Published online Jul 15, 2003. doi: 10.3748/wjg.v9.i7.1469
Revised: December 4, 2002
Accepted: December 22, 2002
Published online: July 15, 2003
AIM: To investigate the density of mast cells (MCs) in human hepatocellular carcinoma (HCC), and to determine whether the MCs density has any correlations with histopathological grading, staging or some baseline patient characteristics.
METHODS: Tissue sections of 22 primary HCCs were histochemically stained with toluidine blue, in order to be able to quantify the MCs in and around the neoplasm using a computer-assisted image analysis system. HCC was staged and graded by two independent pathologists. To identify the sinusoidal capillarisation of each specimen 3 μm thick sections were histochemically stained with sirius red, and semi-quantitatively evaluated by two independent observers. The data were statistically analysed using Spearman's correlation and Student's t-test when appropriate.
RESULTS: MCs density did not correlate with the age or sex of the patients, the serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels, or the stage or grade of the HCC. No significant differences were found between the MCs density of the patients with and without hepatitis C virus infection, but they were significantly higher in the specimens showing marked sinusoidal capillarisation.
CONCLUSION: The lack of any significant correlation between MCs density and the stage or grade of the neoplastic lesions suggests that there is no causal relationship between MCs recruitment and HCC. However, as capillarisation proceeds concurrently with arterial blood supply during hepatocarcinogenesis, MCs may be considered of primary importance in the transition from sinusoidal to capillary-type endothelial cells and the HCC growth.
- Citation: Grizzi F, Franceschini B, Chiriva-Internati M, Liu Y, Hermonat PL, Dioguardi N. Mast cells and human hepatocellular carcinoma. World J Gastroenterol 2003; 9(7): 1469-1473
- URL: https://www.wjgnet.com/1007-9327/full/v9/i7/1469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i7.1469
Mast cells (MCs) have fascinated the biomedical sciences ever since they were first recognised by Paul Ehrlich in the late 1800s'[1]. MCs originate from haematopoietic stem cells in bone marrow[2,3]. MCs circulate in the blood only as progenitors, and it is not until they enter the tissues that they undergo their terminal differentiation into mature cells. MCs provide granule and membrane mediators as well as different cytokines during the course of many human and experimental diseases[4-8]. It has recently been shown that MCs play an important role in antigen presentation to T cells, and that there is a direct interaction between them and the B cells signalling immunoglobulin E (lgE) production. Although a number of studies have shown that MCs play different roles in human tumours[9-26], the exact nature of the relationship between them and HCC has still to be established. The aim of the present study was to investigate MCs density in HCC and compare it with stage and grade of the neoplasia, and some clinical and histopathological characteristics of the patients.
The study was conducted in accordance with the guidelines of the Ethics Committee of the hospital treating the patients (Istituto Clinico Humanitas, Rozzano, Milan, Italy), all of whom were informed of the possible discomforts and risks of surgical treatment.
The study involved 22 primary HCC patients (15 men and 7 women, mean age: 68.22 years, range 48-80 years) who were surgically treated between 1997 and 2001 (Table 1). Tumours were independently graded and staged by two experienced histopathologists. The study was performed on surgical specimens fixed in formalin and embedded in paraffin.
| n | Age | Sex | ALT(UI/l) | AST | Histology | Stage | Grade | Angioinvasivity | HCV-Ab | MCs density(cells/mm2) |
| 1 | 73 | m | 98 | 37 | TR | G1 | pT2 | absent | _ | 0 |
| 2 | 70 | m | 61 | 37 | PS | G2 | pT2 | peritumoural | _ | 0 |
| 3 | 76 | f | 97 | 94 | TR | G2 | pT3 | peritumoural | + | 0 |
| 4 | 59 | m | 169 | 48 | TR | G3 | pT4 | peritumoural | + | 0 |
| 5 | 72 | f | 40 | 29 | TR | G1 | pT3 | peritumoural | nd | 0.0053 |
| 6 | 51 | f | 20 | 32 | TR | G3 | pT3 | peritumoural | _ | 0.0061 |
| 7 | 70 | f | 62 | 66 | C | G1 | pT1 | absent | + | 0.0062 |
| 8 | 67 | m | 108 | 82 | TR | G3 | pT3 | peritumoural | + | 0.0483 |
| 9 | 71 | m | 48 | 64 | PS | G1 | pT2 | peritumoural | _ | 0.0625 |
| 10 | 78 | m | 75 | 116 | TR | G3 | pT2 | absent | + | 0.0726 |
| 11 | 71 | m | 199 | 110 | C | G1 | pT4 | peritumoural | + | 0.253 |
| 12 | 74 | m | 40 | 25 | TR | G1 | pT2 | absent | nd | 0.3846 |
| 13 | 76 | f | 75 | 94 | TR | G2 | pT3 | intratumoural | + | 0.446 |
| 14 | 77 | m | 286 | 95 | TR | G3 | pT3 | absent | + | 0.55 |
| 15 | 63 | m | 97 | 78 | TR | G2 | pT3 | peritumoural | + | 0.625 |
| 16 | 69 | m | 55 | 74 | C | G3 | pT3 | absent | + | 0.894 |
| 17 | 80 | f | 58 | 64 | TR | G3 | pT3 | peritumoural | + | 0.9422 |
| 18 | 51 | m | 103 | 153 | C | G2 | pT2 | absent | + | 1.1 |
| 19 | 68 | f | 79 | 113 | TR | G1 | pT2 | absent | + | 1.15 |
| 20 | 48 | f | 149 | 76 | TR | G1 | pT2 | absent | _ | 2.58 |
| 21 | 73 | m | 116 | 45 | TR | G1 | pT3 | absent | _ | 5.041 |
| 22 | 64 | m | 131 | 70 | C | G2 | pT3 | absent | + | 6.006 |
Sections of 3 μm were cut, mounted on glass slides, de-waxed in xylene and re-hydrated using graded alcohol/water baths. They were then rinsed in distilled water for 5 min and incubated for 30 min at room temperature with a freshly made staining aqueous solution consisting of 0.1% toluidine blue (Sigma Chem. Co., MO, USA) and 0.005% acetic acid. The number of MCs detectable on the whole surface of the available liver sections at a magnification of 200 × was quantified using a computer-assisted image analysis system consisting of an Axiophot light microscope (Zeiss, Germany), a 3-CCD camera (JVC KY-F55BE, Italy), a Pentium 600 computer (Hewlett-Packard, Italy) with an incorporated frame-grabber board (Imascan, USA), and Image-Pro Plus image analysis software (Immaginie Computer, Rho, Italy). The digitized image, which was composed of a variable number of fields tiled together to form a unique final image, represent all of the histological materials available for examination (> 30 mm2 for each slide). The Image Pro-Plus software automatically selected stained MCs as single objects on the basis of similarities in the color of adjacent pixels, the image intensity was the same throughout the study. MCs density (d) was automatically calculated using the formula: d = (number of MCs)/(sample surface), where the sample surface was expressed in μm2.
In order to evaluate the hepatic sinusoid capillarisation of each specimen, a 3 μm section was stained with Direct-red 80 (Sigma Chem. Co., MO, USA; 0.1% in saturated picric acid). The sections were subsequently washed in running tap water for 30 min to remove excess staining, counter-stained in Mayer's hemallum solution, dehydrated, and mounted in Eukitt (Bio-Optica, Milan, Italy). Sinusoid capillarisation was independently scored as strong (+++), moderate (++), weak (+) or absent (0) by two experienced histopathologists.
Linear regression analysis was used to assess the statistical correlation between MCs density and the other histopathological and clinical data. The relationships were determined using Spearman's correlation and Student's t-test when appropriate. Statistical analysis was performed using Statistica software (StatSoft Inc., Tulsa, OK, USA). P < 0.05 was considered statistically significant.
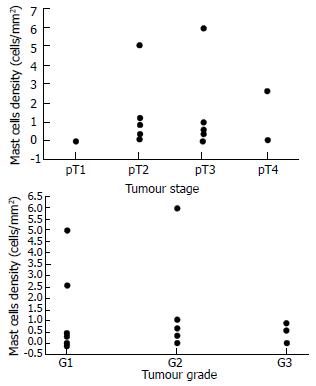
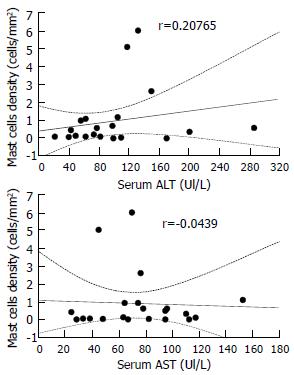
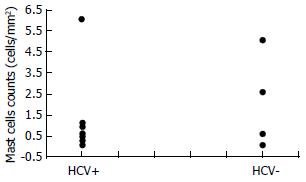
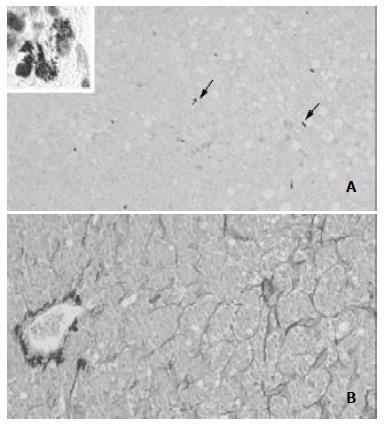
Table 1 shows the baseline characteristics of the 22 patients involved in the study. MCs were strongly identified in 18 cases (82%). They were found in and around the neoplastic tissue. MCs density did not correlate with the patients' age (r = 0.1698). It correlated non-significantly with disease stage (Figure 1A) and disease grade (Figure 1B), nor with serum alanine aminotransferase (ALT, r = 0.20765) or aspartate aminotransferase (AST, r = - 0.0439) (Figure 2). There was no significant difference in the density of MCs between the patients with and without chronic hepatitis C virus disease (Figure 3), but a semi-quantitative evaluation showed that MCs density did correlate significantly with the capillarisation process (Table 2). MCs were found abundantly in tissues showing a higher capillarisation phenomenon (Figure 4).
| MC density | Presence of capillarisation | |||
| Absent | Weak | Moderate | Strong | |
| 0-0.01 | 3 | 2 | 0 | 0 |
| 0.01-0.1 | 3 | 0 | 0 | 0 |
| 0.1-1 | 1 | 5 | 1 | 0 |
| > 1 | 0 | 0 | 2 | 3a |
MCs are a group of long-living cells of bone marrow origin that are commonly found in the skin and the gastrointestinal and respiratory systems. These cells produce, store and release a high number of bioactive mediators, such as TNF-α, IL-1, IL-4, IL-5, IL-6, IL-8, IL-13, NGF, β-FGF and TGF-β, histamine, and the serine proteases, tryptase and chymase[3,4,27].
MCs are known to be present in normal and pathological livers. In normal human livers, there are a few resident MCs located near the portal tracts which are more densely distributed around the biliary tree. Large amounts of MCs have been detected in the fibrotic septa and portal regions in cirrhotic human livers. A number of studies have indicated that MCs are associated with hepatic fibrosis as they promote fibroblast growth, collagen synthesis and may inhibit extracellular matrix degradation by means of inhibitors of metalloproteases (TIMPs)[6,27-32]. MCs density is a valid index of acute liver inflammation[7,8].
MCs were recognized in various human tumours[8,26]. The accumulation of MCs around the neoplasia is a well-known phenomenon in basal cell carcinoma, melanoma, lung, prostate and breast cancer.
Although the functions of intra-tumour MCs are yet unclear (some MCs have been evidenced in close apposition to tumour cells, suggesting the existence of direct cell-to-cell interactions), two main hypotheses have been proposed: The first is that MCs may accelerate tumour growth, invasion and neovascularity. Contrarily, the second is that MCs have cytotoxic activity for some tumours (especially those sensitive to TNF-α), as a result of cytotoxic products or the enhancement of the cytotoxic activation of mainly peri-tumoural eosinophils and macrophages[33-36].
Experimental studies have shown that an increase in MCs density is stimulated by IL-3 and IL-4, thus suggesting that their defensive role against tumour growth may be particularly associated with T cells[37-39]. IL-4 is essential for the triggering of Th2 lymphocytes that they themselves produce to initiate inflammatory cell accumulation and B lymphocyte immunoglobulin class switching to IgE[40].
However, the well documented relationship between MCs and angiogenesis makes the first hypothesis more persuasive[23,27]. Secreting MCs are able to induce and enhance angiogenesis via multiple in part interacting pathways[27]. The supporting evidence includes: (a) The fact that tumour cells produce agents promoting MC chemotaxis. Moreover, most of the tumour-infiltrating MCs exhibit anaphylactic or piecemeal degranulation, indicating that the MCs are activated in situ. (b) MCs degranulation facilitates the endothelial cell invasion in connective tissue. (c) MC heparin stimulates the endothelial cell motility essential for the angiogenic process. (d) The metachromasia that is determined by the presence of highly sulphated proteoglycans in the secretory granules of MCs, is commonly found at the tumour periphery, suggesting the release of vasoactive substances around the tumour. (e) Histamine, VEGF, and certain lipid-derived mediators that induce microvascular hyperpermeability having pro-angiogenic effects.
In the present paper, we showed that HCC contained a higher density of toluidine blue stained MCs, thus suggesting that the recruitment of MCs increases during the development of HCC. However, the results obtained from 22 HCC patients did not indicate any significant correlation between MCs density and disease stage. We hypothesize that the lower density observed in the most severe stage may be due to the massive degranulation of a large number of MCs.
Moreover, the obtained results allow the following conclusions to be drawn: (a) MCs density did not significantly correlate with disease grade, but there was a trend towards a decrease in MCs number as the grade increased. (b) There was no correlation between MCs density and some baseline characteristics of the patients, such as the sex or age of the patients, since the tumour evolves more slowly in older patients, we expected to find a higher density in younger cases. This hypothesis was partially substantiated by an albeit statistically non-significant negative correlation between MCs density and age. This behaviour was in line with theoretical models showing that a large number of biological events were age-dependent [7]. (c) MCs density did not correlate with serum ALT or AST levels. HCV, cirrhosis and HCC were associated with persistent or fluctuating elevations in ALT levels, but did not distinguish among these conditions. (d) There was no significant difference in the density of MCs between patients with and without HCV disease. As it is known that there is a close relationship between HCC and hepatitis C virus infection, it is not surprising that we had more HCV-positive than HCV-negative cases. Nevertheless, MCs density was similar in both groups, suggesting that HCV infection did not increase MC recruitment. (e) The close proximity of MCs and surrounding tumour cells suggested the existence of roles of MCs in the development of HCC, including tumour growth as well as host immunity and stromal reaction. (f) The density of MCs was higher in the specimens showing a greater capillarisation of sinusoidal endothelial cells.
A number of studies have indicated that the sinusoidal endothelial cells tend to show phenotypic changes in the early stage of hepatocarcinogenesis. In HCC, endothelial fenestrae are diminished and basement membrane become thick: due to the fact that as the arterial blood supply for HCC increases, the sinusoidal endothelial cells may form basement membranes, mainly consisting of type IV collagen and laminin, and take on the morphological appearance of capillaries[41-44].
Capillarisation of sinusoids has also constant features such as the appearance of new junctions between endothelial cells, deposition of fibrillary material in Disse's spaces, and flattening of hepatocyte microvilli. Capillarisation of the sinusoid may also cause a disturbance in exchanges of many bioactive substances between the sinusoidal blood and liver cells across the Disse space. Capillarisation, resulting in impairment of microcirculation, subsequently, affecting the exchange of the oxygen and substance of the liver cell seriously, thus brings about or aggravates the damage of liver cell[45]. Capillarisation may contribute to the HCC metastatic process. The angiogenesis of liver metastases may progress stepwise as the metastases enlarge, and capillarisation of sinusoidal endothelium around the liver metastases may occur. A number of papers have suggested that (a) tumour vessels in metastatic liver cancers consist of endothelium, collagenic basement membrane and pericytes, (b) the sinusoids adjacent to tumours undergo capillarisation, and (c) VEGF, a well-demonstrated mediator secreted by MCs may contribute to angiogenesis in metastatic HCC.
It is well documented that heparin, combined to a range of heparin-binding factors such as b-FGF or TGF-β, or other potent factors, such as IL-8 and VEGF, are able to promote neovascularity, and that MC proteases cause loss of the extracellular matrix integrity[27].
All these comments suggest that MCs accumulation at the tumour site may lead to increased rates of tumour vascularity and, consequently, increased rates of tumour growth and metastasis.
In conclusion, since capillarisation proceeds concurrently with arterial blood supply during hepatocarcinogenesis, MCs may be considered as a key element in the process of transition from sinusoidal endothelial cells into capillary-type endothelial cells and concurrently in the development of collagen's basement membrane. MCs mediated capillarisation may thus be of pathogenic significance in tumor growth.
The authors are grateful to prof. Massimo Roncalli and Dr Piergiuseppe Colombo for supplying the HCC specimens, and for their grading and staging classification. They would also like to thank Giorgia Ceva-Grimaidi for her precious technical support.
Edited by Xu XQ and Wang XL
| 1. | Ehrlich P. Beitrage zur kenntniss der quilinfarbunger und ihrer verivendung in der mickroskopischen technik.. Alch Mikros Anat. 1877;13:263-267. [RCA] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 192] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997;61:233-245. [PubMed] |
| 3. | Födinger M, Fritsch G, Winkler K, Emminger W, Mitterbauer G, Gadner H, Valent P, Mannhalter C. Origin of human mast cells: development from transplanted hematopoietic stem cells after allogeneic bone marrow transplantation. Blood. 1994;84:2954-2959. [PubMed] |
| 4. | Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;7:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 274] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033-1079. [PubMed] |
| 6. | Farrell DJ, Hines JE, Walls AF, Kelly PJ, Bennett MK, Burt AD. Intrahepatic mast cells in chronic liver diseases. Hepatology. 1995;22:1175-1181. [PubMed] |
| 7. | Grizzi F, Franceschini B, Barbieri B, Gagliano N, Arosio B, Chiriva-Internati M, Annoni G, Dioguardi N. Mast cell density: a quantitative index of acute liver inflammation. Anal Quant Cytol Histol. 2002;24:63-69. [PubMed] |
| 8. | Grizzi F, Franceschini B, Gagliano N, Moscheni C, Annoni G, Vergani C, Hermonat PL, Chiriva-Internati M, Dioguardi N. Mast cell density, hepatic stellate cell activation and TGF-beta1 transcripts in the aging Sprague-Dawley rat during early acute liver injury. Toxicol Pathol. 2003;31:173-178. [PubMed] |
| 9. | Sari A, Serel TA, Candir O, Oztürk A, Kosar A. Mast cell variations in tumour tissue and with histopathological grading in specimens of prostatic adenocarcinoma. BJU Int. 1999;84:851-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Imada A, Shijubo N, Kojima H, Abe S. Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur Respir J. 2000;15:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Tomita M, Matsuzaki Y, Onitsuka T. Effect of mast cells on tumor angiogenesis in lung cancer. Ann Thorac Surg. 2000;69:1686-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Simak R, Capodieci P, Cohen DW, Fair WR, Scher H, Melamed J, Drobnjak M, Heston WD, Stix U, Steiner G. Expression of c-kit and kit-ligand in benign and malignant prostatic tissues. Histol Histopathol. 2000;15:365-374. [PubMed] |
| 13. | Tóth-Jakatics R, Jimi S, Takebayashi S, Kawamoto N. Cutaneous malignant melanoma: correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum Pathol. 2000;31:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. 2000;33:961-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Demitsu T, Inoue T, Kakurai M, Kiyosawa T, Yoneda K, Manabe M. Activation of mast cells within a tumor of angiosarcoma: ultrastructural study of five cases. J Dermatol. 2002;29:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Yano H, Kinuta M, Tateishi H, Nakano Y, Matsui S, Monden T, Okamura J, Sakai M, Okamoto S. Mast cell infiltration around gastric cancer cells correlates with tumor angiogenesis and metastasis. Gastric Cancer. 1999;2:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Cabanillas-Saez A, Schalper JA, Nicovani SM, Rudolph MI. Characterization of mast cells according to their content of tryptase and chymase in normal and neoplastic human uterine cervix. Int J Gynecol Cancer. 2002;12:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Yavuz E, Güllüoğlu MG, Akbaş N, Tuzlali S, Ilhan R, Iplikçi A, Akhan SE. The values of intratumoral mast cell count and Ki-67 immunoreactivity index in differential diagnosis of uterine smooth muscle neoplasms. Pathol Int. 2001;51:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Elpek GO, Gelen T, Aksoy NH, Erdoğan A, Dertsiz L, Demircan A, Keleş N. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Fukushima N, Satoh T, Sano M, Tokunaga O. Angiogenesis and mast cells in non-Hodgkin's lymphoma: a strong correlation in angioimmunoblastic T-cell lymphoma. Leuk Lymphoma. 2001;42:709-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Tomita M, Matsuzaki Y, Edagawa M, Shimizu T, Hara M, Sekiya R, Onitsuka T. Association of mast cells with tumor angiogenesis in esophageal squamous cell carcinoma. Dis Esophagus. 2001;14:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Erkiliç S, Erbağci Z. The significance of mast cells associated with basal cell carcinoma. J Dermatol. 2001;28:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Ribatti D, Vacca A, Nico B, Crivellato E, Roncali L, Dammacco F. The role of mast cells in tumour angiogenesis. Br J Haematol. 2001;115:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brünner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Reynolds JL, Akhter JA, Magarey CJ, Schwartz P, Adams WJ, Morris DL. Histamine in human breast cancer. Br J Surg. 1998;85:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kankkunen JP, Harvima IT, Naukkarinen A. Quantitative analysis of tryptase and chymase containing mast cells in benign and malignant breast lesions. Int J Cancer. 1997;72:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Norrby K. Mast cells and angiogenesis. APMIS. 2002;110:355-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest. 1997;99:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 218] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Inoue Y, King TE, Barker E, Daniloff E, Newman LS. Basic fibroblast growth factor and its receptors in idiopathic pulmonary fibrosis and lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2002;166:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Armbrust T, Batusic D, Ringe B, Ramadori G. Mast cells distribution in human liver disease and experimental rat liver fibrosis. Indications for mast cell participation in development of liver fibrosis. J Hepatol. 1997;26:1042-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Yamashiro M, Kouda W, Kono N, Tsuneyama K, Matsui O, Nakanuma Y. Distribution of intrahepatic mast cells in various hepatobiliary disorders. An immunohistochemical study. Virchows Arch. 1998;433:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Neubauer K, Saile B, Ramadori G. Liver fibrosis and altered matrix synthesis. Can J Gastroenterol. 2001;15:187-193. [PubMed] |
| 33. | Tani K, Ogushi F, Shimizu T, Sone S. Protease-induced leukocyte chemotaxis and activation: roles in host defense and inflammation. J Med Invest. 2001;48:133-141. [PubMed] |
| 34. | Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23-F26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 282] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, Montecucco C. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur J Immunol. 2002;32:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Dimitriadou V, Koutsilieris M. Mast cell-tumor cell interactions: for or against tumour growth and metastasis. Anticancer Res. 1997;17:1541-1549. [PubMed] |
| 37. | Bradding P, Feather IH, Wilson S, Bardin PG, Heusser CH, Holgate ST, Howarth PH. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993;151:3853-3865. [PubMed] |
| 38. | Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 549] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 39. | Okayama Y, Petit-Frére C, Kassel O, Semper A, Quint D, Tunon-de-Lara MJ, Bradding P, Holgate ST, Church MK. IgE-dependent expression of mRNA for IL-4 and IL-5 in human lung mast cells. J Immunol. 1995;155:1796-1808. [PubMed] |
| 40. | Wilson SJ, Shute JK, Holgate ST, Howarth PH, Bradding P. Localization of interleukin (IL) -4 but not IL-5 to human mast cell secretory granules by immunoelectron microscopy. Clin Exp Allergy. 2000;30:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Kin M, Torimura T, Ueno T, Inuzuka S, Tanikawa K. Sinusoidal capillarization in small hepatocellular carcinoma. Pathol Int. 1994;44:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Yamamoto T, Kaneda K, Hirohashi K, Kinoshita H, Sakurai M. Sinusoidal capillarization and arterial blood supply continuously proceed with the advance of the stages of hepatocarcinogenesis in the rat. Jpn J Cancer Res. 1996;87:442-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Zimmermann A, Zhao D, Reichen J. Myofibroblasts in the cirrhotic rat liver reflect hepatic remodeling and correlate with fibrosis and sinusoidal capillarization. J Hepatol. 1999;30:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Park YN, Yang CP, Fernandez GJ, Cubukcu O, Thung SN, Theise ND. Neoangiogenesis and sinusoidal "capillarization" in dysplastic nodules of the liver. Am J Surg Pathol. 1998;22:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 202] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Huang GW, Yang LY. Metallothionein expression in hepatocellular carcinoma. World J Gastroenterol. 2002;8:650-653. [PubMed] |