Published online Jun 15, 2003. doi: 10.3748/wjg.v9.i6.1231
Revised: January 14, 2003
Accepted: February 11, 2003
Published online: June 15, 2003
AIM: To investigate the effects of KAI1/CD82 on biological behavior of colorectal carcinoma cells.
METHODS: KAI1 cDNA was transfected into highly malignant colorectal carcinoma cell line, LoVo, which had low level of endogenous KAI1 expression, and established stable transfectant clones with high KAI1/CD82 expression. The cell-cell adhesion, cell aggregation, cell-matrix adhesion and cell invasion assay were performed to determine whether KAI1 transfectant could have an effect on proliferation, adhesion and tumor metastasis in comparison with the control transfectant cells.
RESULTS: KAI1 expression did not alter in vitro cell proliferation. But the KAI1 transfectant cells exhibited significantly increased homotypic cell-cell adhesion and cell aggregation in comparison with the control transfectant cells(P < 0.05). Furthermore, KAI1 expression significantly suppressed the cell adhesion to extracellular matrix components and in vitro cell invasion in KAI1-transfected LoVo cells. The data indicated that KAI1 expression significantly suppressed the metastatic potential of KAI1-transfected LoVo cells.
CONCLUSION: Our results suggest that KAI1 might function as a negative regulator of colorectal carcinoma metastasis.
- Citation: Liu L, Wu DH, Li ZG, Yang GZ, Ding YQ. Effects of KAI1/CD82 on biological behavior of human colorectal carcinoma cell line. World J Gastroenterol 2003; 9(6): 1231-1236
- URL: https://www.wjgnet.com/1007-9327/full/v9/i6/1231.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i6.1231
KAI1, a newly identified metastatic suppressor gene for prostatic cancer, is located on human chromosome 11p11.2. It was originally isolated by Dong in 1995[1,2]. KAI1 mRNA, is ubiquitously expressed with abundant expression in the surface epithelium of the major epithelial tissues, including prostate, breast, bladder, and gastrointestinal tract[3,4]. Its protein product, CD82, belongs to TM4SF (transmembrane four superfamily) or TST (tetra span transmembrane) superfamily, many of which, including KAI1, are CD antigens present on the surface of leukocytes[5,6]. Four hydrophobic transmembrane domains and one large extracellular hydrophilic domain containing 3 potential N-glycosylation sites are thought to function in cell-cell and cell-matrix interactions. At least three TM4SF members are implicated in metastasis, including CD9/MRP-1, CD63/ME491, and CD82/KAI1[7]. KAI1 and other TM4SF members have been demonstrated to bind to each other, integrins, and E-cadherin, and other surface molecules to relay extracellular signals to signal transduction pathways that are important in cellular adhesion, invasive motility, and metastasis suppression[8-10].
The role of KAI1 in tumor progression may not be limited to prostatic cancer. It was reported to be important in preventing the development of metastases in a wide variety of human tumor types, including several cancers such as cervical[11], breast[12], pancreatic[13], esophageal[14], and ovarian cancer[15]. Therefore, we transfected KAI1 plasmid into highly malignant colorectal carcinoma cell line, LoVo, and examined cell proliferation, adhesion and invasion. The aim of this study was to determine whether KAI1could suppress the invasive or metastatic ability of colorectal carcinoma cells.
LoVo cell line was derived from human colon adenocarcinoma established from the metastatic nodule resected from a 56-year-old Caucasian man with colon adenocarcinoma in 1972. ECV304 cell line was derived from human umbilical cord transformed endothelium. Both cell lines were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 100 U/mL penicillin/streptomycin. All cells were grown in 5% CO2 humidified atmosphere at 37 °C, and harvested for analysis at 80%-90% confluency.
pCMV-KAI1 plasmid was a generous gift from Carl Barrett. 1.6 kb KAI1cDNA inserting at salI sites was inserted into the xhoI site of pCMV-neo-xhoI vector to construct pCMV-KAI1. This plasmid was transfected into LoVo cell using the cationic polymer transfection reagent(jetPEI trasfection reagent was purchased from Dakewe Biotechnology Company). The transfection proposal was as follows: the cells were plated at 6-well plate 1d before the transfection. 3 μg pCMV-KAI1 plasmid was diluted to 100 μL 150 mM NaCl and 6 μL jetPEI solution was diluted to 100 μL 150 mM NaCl. 100 μL jetPEI solution was added to the 100 μL DNA solution and vortex the solution immediately. Then the mixture was added drop-wise onto the serum containing medium in each well after incubated for 30 min at room temperature. After incubated for 48 h at 37 °C, 5% CO2 in a humidified atmosphere, the transfectants named LoVo-KAI1 were isolated by growing them in G418-containing medium (700 μg/ml) and G418-resistant clones were established in two weeks. The pCMV-neo (purchased from Stratagene Company) vector alone was also transfected into LoVo cells to generate neo transfected control clones, designated as LoVo-neo.
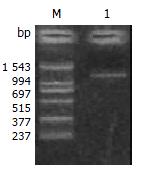
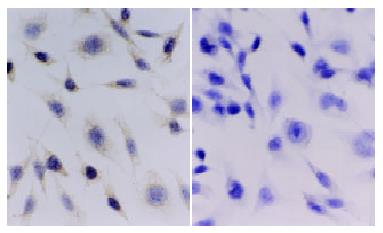
To prepare digoxigenin-labeled KAI1-DNA probes for in situ hybridization, PCR amplification was performed with a forward primer 5'-AGTCCTCCCTGCTGCTGTGTG-3' and reverse primer 5'-TCAGTCAGGGTGGGCAAGAGG-3' and a 1030 bp KAI1 fragment was achieved[16]. The probes was labeled by Dig DNA Labeling and detecting Kit (purchased from Roche Company). Blot hybridization of Dig-labeled probe showed the labeling efficiency. ISH was performed according to the description of the enhanced sensitive ISH detection kit (purchased from Boster Biotechnology Company). Briefly, the cells that were incubated in cover slip for 24 hour and fixed with 95% ethanol were washed with PBS (phosphate-buffered saline) three times. The endogenous peroxidases were blocked by incubating with 0.5% H2O2 for 30 min at room temperature. The cells were subsequently treated for 10 min with triton X-100 and for 40 s with 3% pepsin. At 37 °C the cells were prehybridized for 3 h and hybridized overnight. The final concentrations of the labeled probes were 150 pg/μL. After hybridization, excess probe was removed by washing in 2 × SSC, 0.5 × SSC, 0.2 × SSC respectively. The cells were incubated with an antidigoxigenin antibody conjugated biotin for 120 min at room temperature and then added strept-avidin biotin complex for 30 min. For color reaction, diaminobenzidine (DAB) was used. If the ISH signals were present, the cytoplasm would be full of brown granules. The control was LoVo-neo cell group.
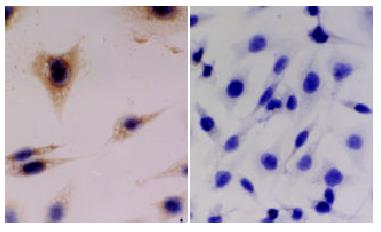
The fixed cells were subjected to immunostaining by using an ultrasensitive S-P technique (Maixin Biotechnology Company). Endogenous peroxidases were blocked by incubating with 0.5% H2O2 for 30 min at room temperature. The cells were subsequently treated for 10 min with triton X-100 and for 40 s with 3% pepsin. The cells were incubated for 30 min at 37 °Cwith normal nonimmune serum before incubation at 4 °C with specific monoclonal KAI1-antibody (BD Pharmingen Technical Company) used at a dilution of 1:100 overnight. The cells were then treated with biotin-conjugated second antibody before adding streptavidin-peroxidase. For color reaction, diaminobenzidine was used. If the IHC signals were present, the cytoplasm and membrane were stained brown. The control was LoVo-neo cell group.
LoVo-KAI1 Cells were prepared by trypsinization. The cells were resuspended at a concentration of 104 cells/mL in RPMI 1640 10% FCS and then 100 μL aliquots were dispensed into 96 well microtiter plates. The cells were incubated for 1, 2, 3, 4, 5, 6 d respectively and MTT assay was performed by adding 20 μL MTT (5 mg/ml) for 4 h. When the MTT incubation was completed, the supernatants were removed. 150 μL dimethyl sulfoxide (DMSO) was added to each well. The A value of each well was measured with a microplate reader at 570 nm 15 min later. All experiments were performed in triplicate. The control was LoVo-neo cell group.
Cell-cell adhesion assays were performed using 96-well plates. The cells were cultured in 96-well plates and in confluence without vacant space. Other homotypic cells were harvested with 0.25% trypsin and resuspended to a density of 105 cells/mL in RPMI-1640 without FCS. Then the cells of 100 μL were added into each well with already cultured homotypic cells and recoverd for 60 min, 90 min at 37 °C. Non-adherent cells were collected by gentle washing with PBS and the washed cells were counted. The number of cell-cell adhesion was calculated using the following formula: The number of cell-cell adhesion = total number of added cells:The number of cells being washed. The experments were performed in triplicate and repeated three times. The control was LoVo-neo cell group.
12-well plate was coated with 1% agar. And then single cell suspension (105/ml) was washed by PBS three time after the cells were detached from the culture flasks with 0.25% trypsin. The cells (1 mL) were added to 12-well plate and incubated at 37 °Cwith rotary shaking (70 r/min) for 5 h. The number of single cell was counted by haemocytometer. The cell-cell aggregation rate was then measured as ((total number of cells-single cells)/(total number of cells)) × 100%.
96-well plates were incubated with 75 μL fibronectin (50 μg/ml) for 45 min and blocked with BSA(10 mg/ml). The cells were harvested as described for cell-cell adhesion assay. The cells (105 cells/ml) of 100 μL were added to each well and allowed to attach 2 h at 37 °C. After washed with PBS twice to withdraw the non-adherent cells, 20 μL MTT was added for 4 h and 200 μL DMSO was added as above. The value of each well was measured with a microplate reader at 570 nm 15 min later. The experments were performed in triplicate. The control was LoVo-neo cell group.
This assay was based on the principle of Boyden chamber[19]. The top and bottom of Boyden chamber (Corning Company) were separated by polycarbonate filter with 8 μm pore size. The top chamber was prepared by coating the filter with 50 mg of diluted matrigel and incubated for 30 min. The bottom chamber was prepared with 5% FBS as a chemoattactant. After 24 h, 48 h incubation, the noninvasive cells were removed with a cotton swab. The cells that had migrated through the membrane and stuck to the lower surface of the membrane were fixed with methanol and stained with hematoxylin. For quantification, the cells were counted under a microscope in five predetermined fields at × 200. The control was LoVo-neo cell group.
ECV304 cells were cultured in cover slip and confluence without vacant space. LoVo-KAI1 cells were harvested with 0.25% trypsin and resuspended to a density of 105 cells/mL in RPMI-1640 with 2% FBS and 105 cells were added to the surface of ECV304 cells. After interaction for 24 h, the cells were washed with PBS and fixed immediately with 95% ethanol. The cells that had migrated through the ECV304 cells and grown under ECV304 cells were counted. For quantification, the cells were counted under a microscope in five random fields at × 200. The experments were performed in triplicate and the control was LoVo-neo cell group.
The comparison was made using Student t-test with a single contrast of LoVo-neo. The P value < 0.05 was considered to be significant.
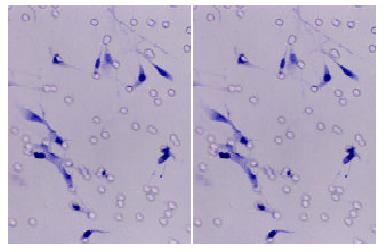
To clarify whether KAI1 could suppress colorectal cancer invasion and metastasis, we transfected KAI1 full-length cDNA into colorectal carcinoma LoVo cell lines. An expression vector was transfected as a negative control. After transfection and G418 selection for two weeks, 10 of each growing clone were picked and screened to confirm the transgene by in situ hybridization and immunohistochemistry. The probes for ISH were about 1000 bp. (Figure 1). The blot hybridization was used to evaluate the efficiency of Dig-labeling probe (Figure 2). KAI1 mRNA expression in LoVo-KAI1 cell was located in the cytoplasm by in situ hybridization. LoVo-KAI1 cells were full of brown granules, but the LoVo-neo cells were negative (Figure 3). KAI1 protein expression was detected by immunohistochemistry and located in the cytoplasm and membrane. LoVo-KAI1 cells displayed positive immunostaining and LoVo-neo cells displayed negative staining (Figure 4). The results suggested that KAI1 was expressed in mRNA and protein level.
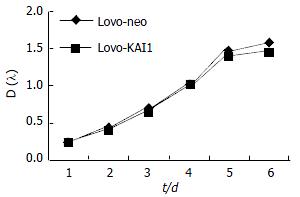
Growth curve showed LoVo-KAI1 cells and LoVo-neo cells existing no difference in proliferation ability (Figure 5). The result demonstrated that KAI1 gene had no effect on cell proliferation ability.
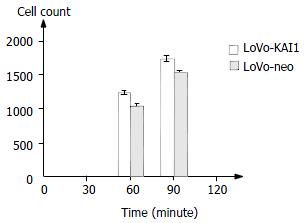
After interaction of homotypic cells in 60 min and 90 min, cell-cell adhesion was analyzed (Figure 6). These data suggested KAI1 gene could increase adhesion of homotypic cells.
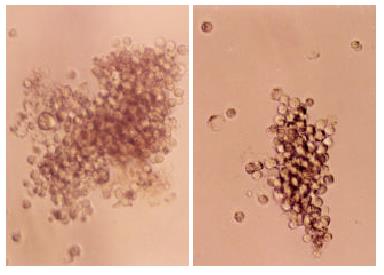
Aggregation observed in the LoVo-KAI1 cells was very different from that of the LoVo-neo cells. The LoVo-KAI1 cells formed large multicellular aggregates containing many cells, whereas the aggregates formed by LoVo-neo cells were much smaller and rarely contained more than a few cells (Figure 7).The number of LoVo-KAI1 cells aggregation (64.8 ± 4.4) was much larger than that of LoVo-neo cells (58.6 ± 3.5) (P < 0.05).
All LoVo-neo cells attached to fibronectin and the surface of fibronectin appeared the phenomena of degradation while most LoVo-KAI1 cells attached to fibronectin and no degradation occurred. MTT assay showed OD570 in LoVo-KAI1 cells was 0.164 ± 0.011 and OD570 in LoVo-neo cells was 0.499 ± 0.023. Attachment of LoVo-neo cells to fibronectin were demonstrated to be much higher compared to attachment of LoVo-KAI1 cells (P < 0.01).
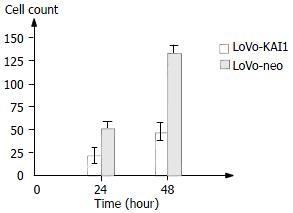
An in vitro cell invasion assay was performed based on the principle of the Boyden chamber assay. The matrigel matrix served as a reconstituted basement membrane in vitro. The number of cells migrating through the Matrigel matrix was counted and the result was presented in Figure 8, Figure 9. The LoVo-KAI1 cells showed significantly reduced invasiveness as compared with LoVo-neo cells (P < 0.01). These data indicated that the enhanced expression of KAI1in LoVo-KAI1 cells was associated with reduced invasive ability.
After cultured for 24 h, some tumor cells had invaded the ECV304 cells and migrated through the monolayer cells of ECV304. The invasive number of LoVo-KAI1 cells was 6.33 ± 1.74 and that of LoVo-neo was 17.67 ± 4.73. The invasive ability of the LoVo-KAI1 cells was significantly reduced compared with that of the LoVo-neo cells (P < 0.05).
Loss of the function of metastasis suppressor genes is an important step in the progression of a tumor type. KAI1 is thought to be one of such metastasis suppressor genes because it has been shown to suppress the ability of human prostatic cancer cells to metastasize when the tumor is transplanted into nude mice[1] and because KAI1 mRNA expression is reduced in advanced pancreatic cancer[20], it enables the pancreatic cancer cells to spread to lymph nodes and distant organs. Furthermore, transfer of the KAI1 gene into mammary cancer cells has been shown to lead to suppression of their metastatic potential, whereas their primary tumor growth is not affected.
In our previous studies, KAI1 gene expression at protein level was inversely correlated with the metastatic potential of some established colorectal carcinoma cell lines. Maurer et al[21] also demonstrated that the progression of colorectal cancer was associated with decreased expression of KAI1. To further elucidate the effect of KAI1 on colorectal carcinoma cell behavior, we transfected KAI1 cDNA into highly malignant colon adenocarcinoma cell lines, LoVo, which have low level of endogenous KAI1 expression. Consistent with the studies of prostatic cancer[2], breast cancer[12] cells by Dong and Yang, our data also indicated that KAI1 expression did not alter in vitro cell proliferation.
To determine if KAI1 expression was reflected in changes of intercellular adhesion, we used cell-cell adhesion assay and cell-cell aggregation assay. We found KAI1 could increase homotypic cells adhesion and the transfectants formed large multicellular aggregates than non-transfected cells. In terms of cell-matrix, our results showed that KAI1 could reduce the transfected cells adhesion to fibronectin as compared with the controls. Member of the TM4SF family of proteins associated with each other and specific integrins[22], suggesting that they might be involved in cell-cell and cell-matrix interactions. Current thinking suggests that these interactions are involved in the formation of multiprotein complexes within the cell membrane that include cell surface receptors, specific integrins and TM4SF proteins. Consequently, a reduced expression of TM4SF proteins might reasonably be expected to have profound effects on cell-cell and cell-matrix interactions. An important avenue of further investigation will be to determine precisely the functions and location of TM4SF-integrin complexes. In this context, Berditchevski[23] has recently provided evidence that TM4SF can modulate integrin signaling and point to a mechanism by which TM4SF proteins regulate cell adhesion.
Our data showed KAI1 could decrease invasive ability in the transfected cells to a reconstituted basement membrane and endothelium compared to the controls. And there is also accumulated evidence that KAI1 is involved in the reduction of invasive tumor cells. Imai demonstrated that decreased KAI1 gene expression might be associated with increased invasive ability of oral squamous cell carcinoma[24]. Takaoka's study also suggested that reduced KAI1 gene expression might contribute to the invasiveness and metastatic ability of colon cancer cells[25]. How does reduced TM4SF expression cause changes of invasive ability in tumor cells It was hypothesized that tetraspanins might be implicated in the assembly of integrin-containing signaling complexes, thus modulating the function of integrin receptors in cell migration[26]. Sugiura's results indicated that integrin-tetraspanin protein complexes played an important role in regulating protrusive activity of the tumor cells and contributed to ECM-induced production of MMP-2, and as a consequence, the invasive ability of cells[27].
Our in vitro study are consistent with in vivo observations that reduced KAI1 expression is associated with invasive human colorectal carcinoma. Muneyuki and Lombardi found that KAI1 expression was closely correlated with clinicopathological factors for colorectal cancers and appeared to be a useful prognostic marker[28-30].
KAI1 has been extensively studied for its involvement in the progression of different human cancers. The mechanism of down-regulation is also analyzed although it exists much debate. Mashimo recently found a putative p53 consensus-binding site within the promoter region of KAI1 and demonstrated that the loss of p53 function, which is commonly observed in many types of cancer, leads to the down-regulation of KAI1 gene, which may result in the progression of metastasis[31]. But Uzawa's data suggest that the down-regulation of KAI1 is not associated with either mutation, allelic loss, methylation of the promoter, or p53 regulation[32]. Our previous study also demonstrated that mutation of KAI1 gene, methylation of CpG islands and abnormality of p53 were not related to low expression of KAI1.
Our results demonstrated a significantly increased adhesion to homotypic cells and decreased adhesion to extracellular matrix components and lower level of invasiveness. In conclusion, KAI1 has a close relationship with tumor adhesion and invasion and the effect of KAI1 appears to be directly targeted on tumor metastasis. Through our studies, we wish colorectal carcinoma cells would be more homotypical adheson, less adhesive to matrix, less invasive[33] that are necessary for metastasis.
Edited by Wu XN
| 1. | Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 546] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Dong JT, Isaacs WB, Barrett JC, Isaacs JT. Genomic organization of the human KAI1 metastasis-suppressor gene. Genomics. 1997;41:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Petty HR, Worth RG, Todd RF. Interactions of integrins with their partner proteins in leukocyte membranes. Immunol Res. 2002;25:75-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428-442. [PubMed] |
| 5. | White A, Lamb PW, Barrett JC. Frequent downregulation of the KAI1(CD82) metastasis suppressor protein in human cancer cell lines. Oncogene. 1998;16:3143-3149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Okochi H, Mine T, Nashiro K, Suzuki J, Fujita T, Furue M. Expression of tetraspans transmembrane family in the epithelium of the gastrointestinal tract. J Clin Gastroenterol. 1999;29:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Adachi M, Taki T, Konishi T, Huang CI, Higashiyama M, Miyake M. Novel staging protocol for non-small-cell lung cancers according to MRP-1/CD9 and KAI1/CD82 gene expression. J Clin Oncol. 1998;16:1397-1406. [PubMed] |
| 8. | Rubinstein E, Le Naour F, Lagaudrière-Gesbert C, Billard M, Conjeaud H, Boucheix C. CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. Eur J Immunol. 1996;26:2657-2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 294] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Bienstock RJ, Barrett JC. KAI1, a prostate metastasis suppressor: prediction of solvated structure and interactions with binding partners; integrins, cadherins, and cell-surface receptor proteins. Mol Carcinog. 2001;32:139-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Hashida H, Takabayashi A, Tokuhara T, Taki T, Kondo K, Kohno N, Yamaoka Y, Miyake M. Integrin alpha3 expression as a prognostic factor in colon cancer: association with MRP-1/CD9 and KAI1/CD82. Int J Cancer. 2002;97:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Liu FS, Chen JT, Dong JT, Hsieh YT, Lin AJ, Ho ES, Hung MJ, Lu CH. KAI1 metastasis suppressor gene is frequently down-regulated in cervical carcinoma. Am J Pathol. 2001;159:1629-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Yang X, Wei LL, Tang C, Slack R, Mueller S, Lippman ME. Overexpression of KAI1 suppresses in vitro invasiveness and in vivo metastasis in breast cancer cells. Cancer Res. 2001;61:5284-5288. [PubMed] |
| 13. | Friess H, Guo XZ, Tempia-Caliera AA, Fukuda A, Martignoni ME, Zimmermann A, Korc M, Büchler MW. Differential expression of metastasis-associated genes in papilla of vater and pancreatic cancer correlates with disease stage. J Clin Oncol. 2001;19:2422-2432. [PubMed] |
| 14. | Miyazaki T, Kato H, Shitara Y, Yoshikawa M, Tajima K, Masuda N, Shouji H, Tsukada K, Nakajima T, Kuwano H. Mutation and expression of the metastasis suppressor gene KAI1 in esophageal squamous cell carcinoma. Cancer. 2000;89:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Liu FS, Dong JT, Chen JT, Hsieh YT, Ho ES, Hung MJ. Frequent down-regulation and lack of mutation of the KAI1 metastasis suppressor gene in epithelial ovarian carcinoma. Gynecol Oncol. 2000;78:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Tagawa K, Arihiro K, Takeshima Y, Hiyama E, Yamasaki M, Inai K. Down-regulation of KAI1 messenger RNA expression is not associated with loss of heterozygosity of the KAI1 gene region in lung adenocarcinoma. Jpn J Cancer Res. 1999;90:970-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Whittard JD, Akiyama SK. Activation of beta1 integrins induces cell-cell adhesion. Exp Cell Res. 2001;263:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Jackson P, Kingsley EA, Russell PJ. Inverse correlation between KAI1 mRNA levels and invasive behaviour in bladder cancer cell lines. Cancer Lett. 2000;156:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Itoh F, Yamamoto H, Hinoda Y, Imai K. Enhanced secretion and activation of matrilysin during malignant conversion of human colorectal epithelium and its relationship with invasive potential of colon cancer cells. Cancer. 1996;77:1717-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Guo X, Friess H, Graber HU, Kashiwagi M, Zimmermann A, Korc M, Büchler MW. KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res. 1996;56:4876-4880. [PubMed] |
| 21. | Maurer CA, Graber HU, Friess H, Beyermann B, Willi D, Netzer P, Zimmermann A, Büchler MW. Reduced expression of the metastasis suppressor gene KAI1 in advanced colon cancer and its metastases. Surgery. 1999;126:869-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Shibagaki N, Hanada Ki, Yamashita H, Shimada S, Hamada H. Overexpression of CD82 on human T cells enhances LFA-1/ICAM-1-mediated cell-cell adhesion: functional association between CD82 and LFA-1 in T cell activation. Eur J Immunol. 1999;29:4081-4091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Berditchevski F, Odintsova E. Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J Cell Biol. 1999;146:477-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 238] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Imai Y, Sasaki T, Shinagawa Y, Akimoto K, Fujibayashi T. Expression of metastasis suppressor gene (KAI1/CD82) in oral squamous cell carcinoma and its clinico-pathological significance. Oral Oncol. 2002;38:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Takaoka A, Hinoda Y, Satoh S, Adachi Y, Itoh F, Adachi M, Imai K. Suppression of invasive properties of colon cancer cells by a metastasis suppressor KAI1 gene. Oncogene. 1998;16:1443-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Hemler ME, Mannion BA, Berditchevski F. Association of TM4SF proteins with integrins: relevance to cancer. Biochim Biophys Acta. 1996;1287:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Muneyuki T, Watanabe M, Yamanaka M, Shiraishi T, Isaji S. KAI1/CD82 expression as a prognosic factor in sporadic colorectal cancer. Anticancer Res. 2001;21:3581-3587. [PubMed] |
| 28. | Mikami T, Ookawa K, Shimoyama T, Fukuda S, Saito H, Munakata A. KAI1, CAR, and Smad4 expression in the progression of colorectal tumor. J Gastroenterol. 2001;36:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Lombardi DP, Geradts J, Foley JF, Chiao C, Lamb PW, Barrett JC. Loss of KAI1 expression in the progression of colorectal cancer. Cancer Res. 1999;59:5724-5731. [PubMed] |
| 30. | Sugiura T, Berditchevski F. Function of alpha3beta1-tetraspanin protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2). J Cell Biol. 1999;146:1375-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Mashimo T, Watabe M, Hirota S, Hosobe S, Miura K, Tegtmeyer PJ, Rinker-Shaeffer CW, Watabe K. The expression of the KAI1 gene, a tumor metastasis suppressor, is directly activated by p53. Proc Natl Acad Sci U S A. 1998;95:11307-11311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Uzawa K, Ono K, Suzuki H, Tanaka C, Yakushiji T, Yamamoto N, Yokoe H, Tanzawa H. High prevalence of decreased expression of KAI1 metastasis suppressor in human oral carcinogenesis. Clin Cancer Res. 2002;8:828-835. [PubMed] |
| 33. | Geradts J, Maynard R, Birrer MJ, Hendricks D, Abbondanzo SL, Fong KM, Barrett JC, Lombardi DP. Frequent loss of KAI1 expression in squamous and lymphoid neoplasms. An immunohistochemical study of archival tissues. Am J Pathol. 1999;154:1665-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |