Published online May 15, 2003. doi: 10.3748/wjg.v9.i5.996
Revised: December 23, 2002
Accepted: January 2, 2003
Published online: May 15, 2003
AIM: To investigate the expression of recombinant HBsAg (rHBsAg) in transgenic cherry tomatillo in order to explore the feasibility of producing HBV oral vaccine with cherry tomatillo by animal immune tests.
METHODS: The recombinant plant expression vector containing HBsAg gene was constructed. Mediated with Agrobacterium tumefaciens, HBsAg gene was transferred into cotyledons of cherry tomatillo. Transformed cherry tomatillos were obtained through hygromycin delay-selection. Integrated DNA in transgenic cherry tomatillo was confirmed by hygromycin resistance selection, Gus detection, polymerase chain reaction (PCR) and dot blotting analysis. Antigenicity of rHBsAg was examined by ELISA and the immunogenicity of rHBsAg derived from transgenic cherry tomatillo tissues was confirmed by oral feed of transformed tissues to BALB/c mice primed with commercial HBV vaccines. Specific antibody titers in mice’s serum were examined by ELISA every week.
RESULTS: By far, 10 positive lines of transgenic cherry tomatillos containing HBsAg gene were obtained. Among different organs of the same transgenic cherry tomatillo, level of rHBsAg expressed in leaves was the highest with the yield up to 300 ng/g fresh weight. And the rHBsAg expression level in fruits was about 10 ng/g fresh weight. In animal immune tests, oral delivery with transgenic tissues to mice primed with commercial vaccine instead of naive mice resulted in significant immune response.
CONCLUSION: The result of this animal immune test indicated the rHBsAg derived from transgenic cherry tomatillo possessed normal immunogenicity. This work demonstrated the feasibility to generate oral immunogenic rHBsAg in transgenic cherry tomatillo, and would provide some experimental approach for the production of low-cost oral vaccines using transgenic cherry tomatillo in large scale.
- Citation: Gao Y, Ma Y, Li M, Cheng T, Li SW, Zhang J, Xia NS. Oral immunization of animals with transgenic cherry tomatillo expressing HBsAg. World J Gastroenterol 2003; 9(5): 996-1002
- URL: https://www.wjgnet.com/1007-9327/full/v9/i5/996.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i5.996
Hepatitis B virus infection is one of the most widespread viral infections of humans, especially in China. HBV DNA were found in most of newborns of HBsAg positive mothers[1]. HBV vaccines have prevented numerous infections. However, administration of vaccine intramuscularly causes some pain, it is not accepted widely, especially in children. In recent years, a novel production system of vaccines-"edible vaccines" or "oral vaccines" is developed. Oral vaccines can serve multiple immunization priorities, including simplicity of use, increase in compliance (as a result of increased comfort of delivery), enhanced immune responses at mucosal sites, and stimulation of humoral immunity[2-5].
The use of transgenic vegetables and fruits for the expression and delivery of recombinant protein antigens as edible vaccines has become an attractive topic for plant molecular biologists. Plants are potential source of antigen protein that is not dependent upon process technology to ensure protein folding and particle assembly. In addition, a plant-based antigen protein expression system makes possible the testing of an oral immunization strategy by simply feeding the plant tissues. So far, the production of oral vaccines in transgenic plant has focus on viral and bacterial subunit antigen, including E. coli heat-labile enterotoxin B subunit (LT-B)[6-12], cholera toxin B subunit (CT-B)[13,14], hepatitis B surface antigen (HBsAg)[15-21], Norwalk virus capsid protein (NVCP)[22,23], rabies virus glycoprotein[24], etc. The results of oral immune tests in human bodies with transgenic potato and lettuce expressing rHBsAg have been reported, and the special antibody was detected in serum of the volunteers who were given edible tissues of transgenic plant[25]. As we know, potato and lettuce could not be eaten in raw in certain population. But cooking process would destroy the target protein and affect its immunogenicity. So the plant acceptor should not only be easy to handle in genetic constructs, but also be able to be consumed in raw. In our study, we chose cherry tomatillo as the plant system for producing recombinant antigen.
Tomatillo, Physalis ixocarpa Brot. is grown in Mexico annually. It is a good source of vitamin C. The flesh of tomatillo is solid and seedy. And the pulp is glutinous, a little sweeter than tomato, and the flavor is somewhat similar to green apple. It is a key ingredient of many Mexican recipes, raw or cooked. Ripe fruits can be eaten raw, in sandwiches and salads. As an important commercial vegetable crop in the diets of Mexicans and Central Americans, tomatillo has been known by more and more researchers. The aim of this study that chose tomatillo as the plant acceptor was to investigate the feasibility of producing HBV oral vaccine with transgenic cherry tomatillo.
Plant materials Cherry tomatillo (Physalis ixocarpa Brot.). The seeds were obtained from Xiamen ITG Seed IMP&EXP CO., LTD.
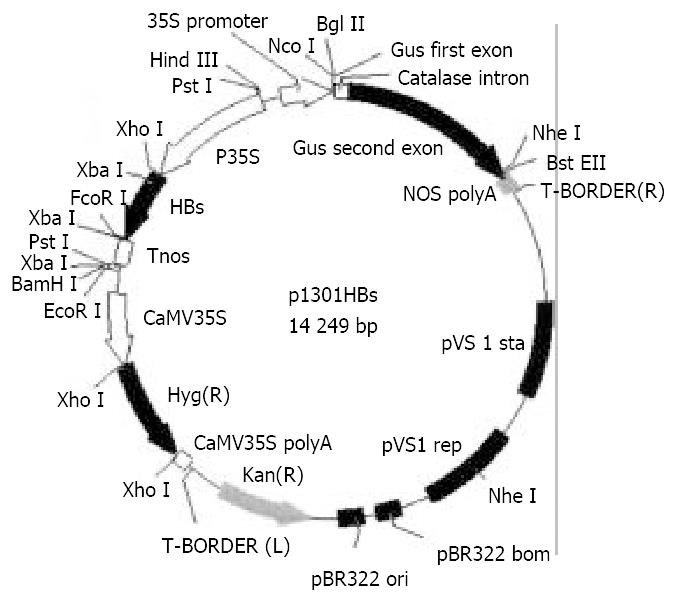
Bacteria and plasmidsAgrobacterium tumefaciens strain EHA105, was kindly provided by Prof. Zhang Qi-fa, Huazhong Agricultural University. p1301HBs, recombinant mini Ti plasmid of the binary expression vector (containing hygromycin-resistant gene, kanamycin-resistant gene, Gus gene and HBsAg-S gene), was constructed in our lab[20,21].
Animals in immune tests BALB/c mice aged 4-6 weeks were purchased from the Center of Cancer Research, Xiamen University.
Construction of plant expression vector A 0.8 kb DNA fragment containing HBsAg-S gene was obtained by a PCR-based assembly from patient’s serum and inserted into pBPFΩ7 between the constitutively-expressed cauliflower mosaic virus (CaMV) 35S promoter and Nos terminator at the Xho I/EcoR I site by substituting the Ω gene to form pBHBs, then the 2.4 kb DNA fragment containing the HBsAg-S gene, the CaMV35S promoter and the nos terimator was cleaved with PstI from pBHBs and ligated into the binary plant vector pCAMBIA1301 (containing hygromycin-resistant gene, kanamycin-resistant gene, Gus gene) digested by Pst I to give the resulting plasmid, p1301HBs (Figure 1).
Plant transformation and regeneration p1301HBs was introduced into Agrobacterium tumefaciens strain EHA105 directly by the freeze-thaw method with slight modifications. Subsequently, Agrobacterium tumefaciens carrying p1301HBs was used to transform cherry tomatillo cotyledons disks. Ten-days post germination cotyledons were excised from in vitro-germinated seedlings, and co-cultivated for 48-72 h with an overnight-grown culture of Agrobacterium tumefaciens carrying p1301HBs on a shooting medium containing 2 mg/l 6-benzyladenine (6-BA) and 0.2 mg/l indole-3-acetic acid (IAA). Cotyledons were then rinsed with sterilized water added 500 mg/l cefotaxime to kill the A. tumefaciens on the surface of explants, blotted dry on a sterilized paper towel, and placed onto a shooting medium added 500 mg/l cefotaxime for recovery. After seven days’ recovery period, the explants were transferred to a shooting medium added 300 mg/l cefotaxime and 25 mg/l hygromycin to select transgenic progeny. About eight weeks later, hygromycin-resistant shoot regenerants were removed to a rooting medium containing 0.2 mg/l IAA and 25 mg/l hygromycin. Rooted plantlets were acclimatized and transferred to a greenhouse for fruiting.
Detection of expression activity of reporter gene-Gus gene
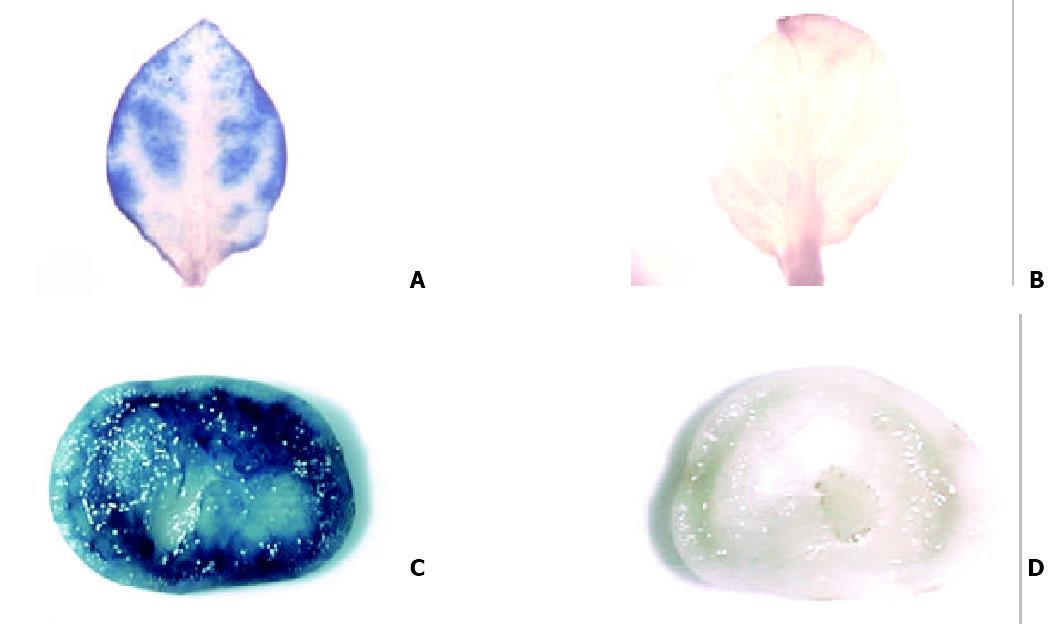
Histochemical quantification of Gus activity was performed. Different tissues were cut from transgenic plantlets and immerged into Gus reaction buffer (889 mg/l X-Gluc, 100 mg/l chloramphenicol, 50 mM NaH2PO4, 1 ‰Triton-X100, 20% methanol, pH7.0-8.0). After incubation at 37 °C for 24 h, the tissues were bleached with absolute alcohol, then observed and photographed under the dissecting microscope. Tissues from non-transformed cherry tomatillo were used as negative control.
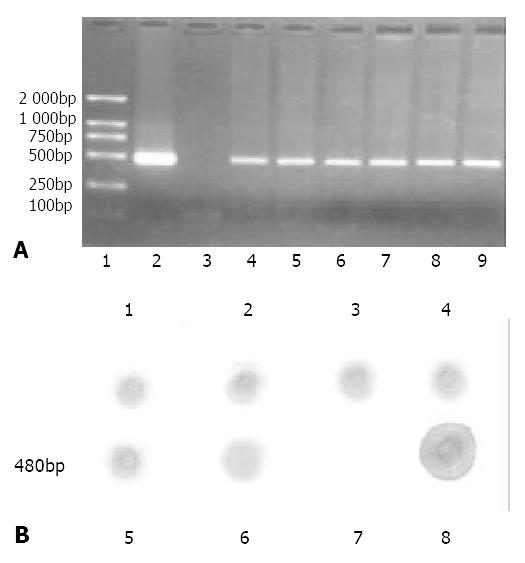
PCR amplification of plant genomic DNA Transgenic plants were checked for the presence of target gene in leaf tissue using PCR and dot-blot analysis. Genomic DNA samples extracted from leaves of regenerated cherry tomatillos plants by the CTAB method[26] were used as templates in PCR reactions using the following primers: forward primer 5’-ATGGAGAACACAACATC-3’; reverse primer 5’-GGATCCTTTTGCGGAAGCCCA-3’, such that amplification would yield a PCR product of 480 bps. The DNA was denatured at 94 °C for 10 min. Forty cycles of PCR were performed at: 94 °C for 50 sec, 45 °C for 45 sec, 72 °C for 50 sec; followed by a 72 °C incubation for 7 min. A positive control (p1301HBs) and a negative control (DNA from non-transformed tomatillo leaves) were included in this experiment. Analysis of PCR products was carried out by loading samples onto a 1.2% agarose gel containing ethidium bromide (EB), followed by electrophoresis and visualization via ultraviolet transillumination. A molecular weight standard (DL-2000, Takara) was used.
Dot blotting The denatured genomic DNA samples were loaded onto a nitrocellulose membrane (Millipore). A positive control (p1301HBs) and a negative control (DNA from non-transformed tomatillo leaves) were included in this experiment. Pre-hybridization was followed by DNA hybridization using a DIG-labelled probe generated from the same 480 bps fragment that was amplified from p1301HBs. Pre-hybridization and hybridization conditions were both at 42 °C. After washing procedure, the membrane was incubated with alkaline phosphatase-conjugated anti-DIG antibody for 2 h at room temperature. The membrane was finally soaked in the color solution at room temperature for up to 4 h in the dark, and the reaction was stopped by rinsing the membrane in distilled water when satisfactory color was observed.
ELISA Different organs of Cherry tomatillo were harvested, frozen in liquid N2, and ground to a fine power. The power was suspended in extraction buffer (50 mM Tris-HCl, 0.029% NaN3, pH9.5) at room temperature overnight. The slurry was then centrifuged at 12000 rpm for 10 min at 4 °C, and supernatants were tested for HBsAg by ELISA (Double Antibody sandwich-ELISA Kit, provided by Beijing Wantai Biological Medicine Co.) following the corresponding protocol. Total soluble protein was quantified using Molecular Biology Assistant (MBA2000, Perkin-Elmer Co.).
Oral primary immunization Two four-week-old BALB/c mice were fed 20 g transgenic tomatillo tissues (containing the equivalent of approximate 1 µg HBsAg) every day. Two control mice were fed wild-type tomatillo tissues. After four weeks, two groups of mice were all boosted parenterally with a subimmunogenic dose (containing 0.5 µg HBsAg) of commercial vaccine (Chuangchun Institute of Biological Products).
Oral booster immunization Four four-week-old BALB/c mice were administered parenterally with commercial vaccine (containing 2 µg HBsAg) as primary immunization. Once the serum antibody levels of the mice decreased apparently, three mice were fed transgenic cherry tomato tissues (20 g/day, containing the equivalent of approximately 1 µg HBsAg) and negative control one was fed wild-type tomatillo tissues.
Antibody assays Mice sera were collected once every week and evaluated for anti-HBsAg-specific antibodies using a commercially available enzyme immunoassay (EIA) diagnostic kit (Double Antigen sandwich-ELISA Kit, Beijing Wantai Biological Medicine Co.).
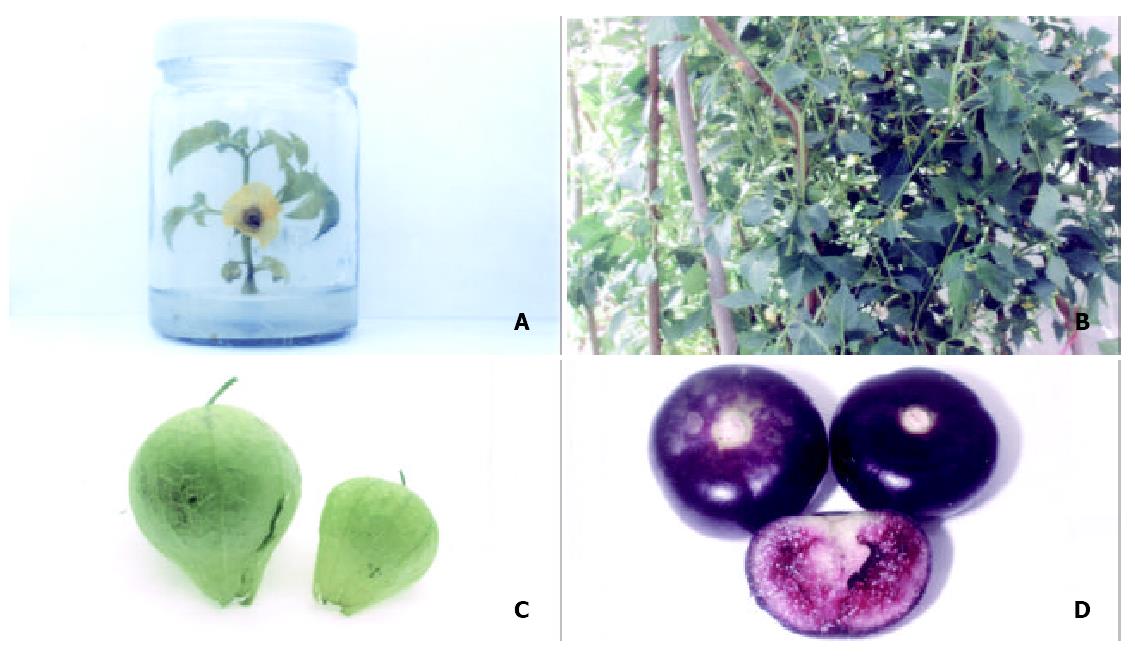
The plasmid for expression of HBsAg in plants (p1301HBs, Figure 1) was introduced into Agrobacterium tumefaciens and used in transformation experiments. Hygromycin incorporated into tissue culture media allowed for selection of transformed callus tissue from which shoots and thereafter mature cherry tomatillo plants were regenerated over a period of about 2-3 months. Ten independent hygromycin-resistant lines of transgenic plants were generated finally. Some lines grew poorly in tissue culture. Such effects could result from insertional mutagenesis. Four lines appearing similar in morphology to wild-type plants were transferred to a greenhouse for fruiting (Figure 2). And F1 progeny of one line was obtained.
The transformed plants, containing Gus gene as the report gene, were tested for the Gus activity (Figure 3). The results demonstrated that Gus gene was stably integrated into nuclear chromosomal DNA of tomatillo and different organs all presented a remarkably high Gus activity.
To verify that these plants contained the target gene, genomic DNA extracted from young leaves was subjected to PCR amplification and the PCR products were electrophoresed through agarose. Samples containing the target gene were identified by the presence of an amplified product of 480 bp, representing a fragment of the HBsAg-S coding sequence in p1301HBs. The results obtained demonstrated the presence of an amplified product of the expected size in all the hygromycin-resistant plants tested, which was absent in non-transformed plants (Figure 4 A).
To further confirm that HBsAg coding gene was stably integrated into nuclear chromosomal DNA of transgenic cherry tomatillo, genomic DNA was analyzed by dot blotting, which showed that all the transgenic samples had the same hybridization signals as the positive control (p1301HBs) (Figure 4 B).
Plants positive in PCR and dot blotting were further tested by ELISA to detect and quantitatively analyze the target protein. The rHBsAg levels in different organs from a representative one of several tactile lines are presented in Table 1. The results indicated that the expression levels of rHBsAg varied among different organs of the same plant. Apparently, the amount of rHBsAg produced in leaf was much higher than any of the other organs. rHBsAg protein levels in fruit and stem were in a similar range. However, rHBsAg/TSP (total soluble protein) value of fruit was somewhat higher than that of leaf. No significant variation was observed among the rHBsAg/TSP values of the same kind of organs in different individuals reproduced from the same line, but striking difference in the absolute expression levels of rHBsAg was observed and the level of TSP in leaves of the plantlets in tissue culture was significantly lower than that in leaves of the plants in greenhouse. The difference might be due to growth condition of these plants.
| Plant No. | Organ | Total soluble protein (mg/g fresh weight) | Content of HBsAg (ng/g fresh weight) | HBsAg/TSP (1/1000000) |
| 1# | Leaf | 31.6 | 300 | 9 |
| Stem | 5.8 | 15 | 3 | |
| Fruit | 0.7 | 7 | 10 | |
| 2# | Leaf | 30.0 | 240 | 8 |
| Stem | 5.0 | 9 | 2 | |
| Fruit | 0.6 | 6 | 10 | |
| 3# | Leaf | 27.3 | 210 | 8 |
| Stem | 3.6 | 7 | 2 | |
| Fruit | 0.6 | 6 | 10 | |
| 4# | Leaf | 11.3 | 90 | 8 |
| Stem | 2.6 | 5 | 2 | |
| CK | Leaf | 28.4 | - | - |
| Stem | 4.7 | - | - | |
| Fruit | 0.6 | - | - |
The rHBsAg levels in leaves of tissue-cultured plantlets of four different lines were shown in Table 2. ELISA analysis demonstrated that rHBsAg was expressed in these different lines ranging from 21 to 90 ng/g fresh weight.
| Line No. | Total soluble protein (mg/g fresh weight) | Content of HBsAg (ng/g fresh weight) | HBsAg/TSP (1/1000000) |
| 1# | 16.0 | 45 | 3 |
| 2# | 15.5 | 24 | 2 |
| 3# | 12.6 | 21 | 2 |
| 4# | 13.1 | 90 | 7 |
| CK | 13.7 | - |
The immunogenicity of plant-derived rHBsAg was tested by oral immunizing BALB/c mice with transgenic tissues from plant line with highest production of rHBsAg. Then vaccinated mice were bled weekly, and the sera were assessed for the presence of specific antibodies by ELISA.
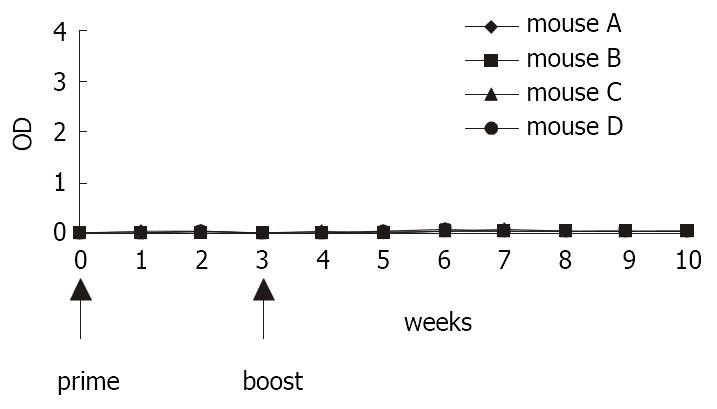
Oral immunization of two naive mice by feeding transgenic tissues did not evoke primary antibody within four weeks. Then mice in two groups were both injected with a subimmunogenic dose (0.5 μg HBsAg) of commercial HBV vaccine via an intramuscularly route as booster. However, none of the mice developed measurable specific serum antibodies in the following examination. There was no significant difference between serum antibody titers of the mice primed with transgenic tissues and those of the control mice in this experiment (Figure 5).
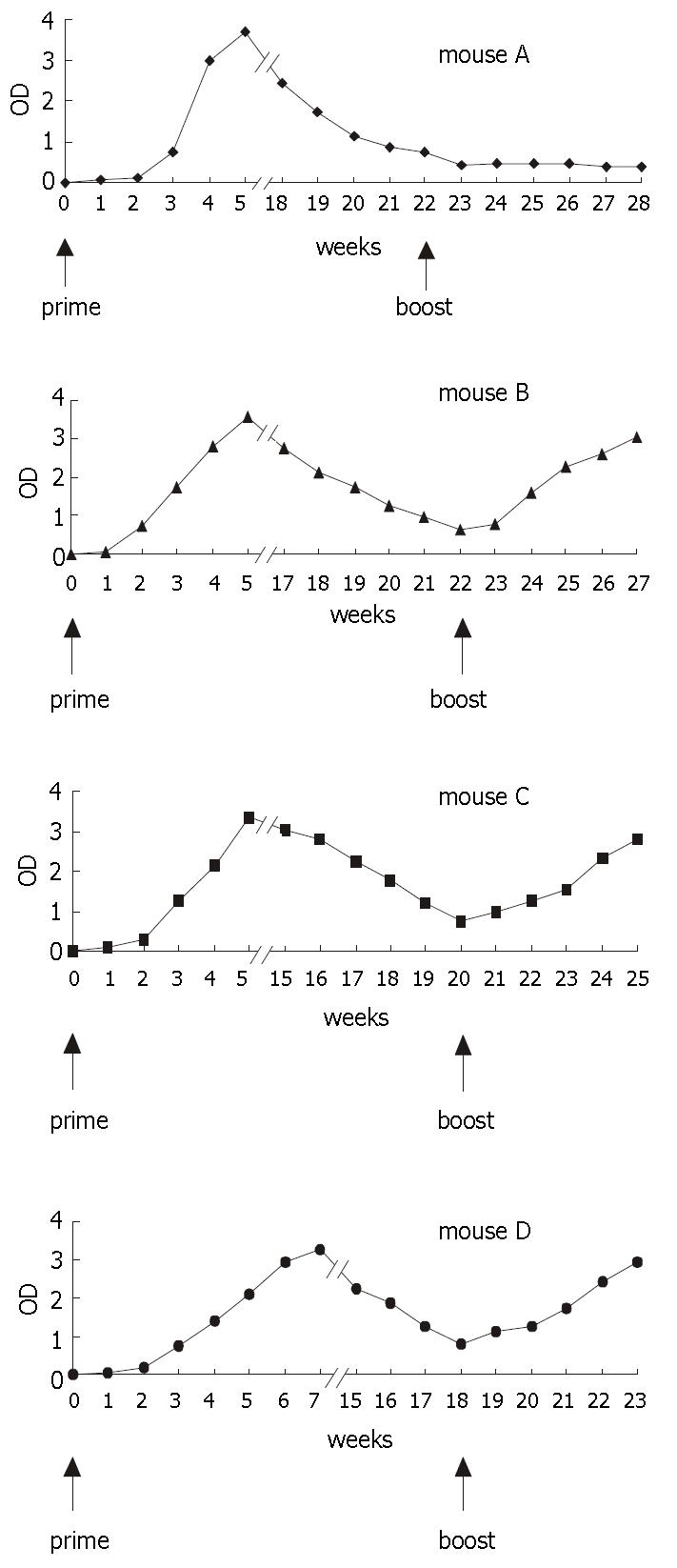
After a single dose (2 μg HBsAg) of commercial vaccine as priming, titers of serum antibody in mice rose sharply in response to antigen initially and then declined. Three mice primed were orally immunized with tissues of the transgenic tomatillo tissues as booster, and elicited an immediate high-level recall antibody response within the following 3-5 weeks. Antibody titers in serum were augmented significantly. In comparison, one negative control mouse fed with wild type tomatillo tissues did not showed any booster response. Serum titers of antibody remained same (Figure 6).
We concluded that immune memory cells were established in mice following parenteral immunization of commercial vaccine and a recall response was elicited by oral delivery of HBsAg in transgenic tissues.
Our results showed that tomatillo-derived rHBsAg had same immunogenicity as commercial vaccine in stimulating production of HBsAg-specific antibodies in animal. The present experiment was limited by the total amount of plant tissues that mice ingested in 24 h and the amount of rHBsAg produced in the transgenic tissues. Absence of expected anti-HBsAg response in the oral priming study might be due to inadequate immunization schedule, such as low doses of antigen, or low sensitivity of the detection system and interference of plant material with development of an immune response. In contrast, active immune response to orally administrated antigens in parenterally primed animals might be due to altered antigen processing in favoring of active antibody production rather than oral immunotolerance.
Oral vaccination using transgenic plants is a viable approach to the development of a novel HBV vaccine. Availability of the vaccine in an edible form as a fruit or vegetable would enhance vaccination coverage by providing an inexpensive, easy to deliver and relatively heat-stable package for distribution. Such a vaccine would have the potential to enable rates of vaccination to reach the targets required for global eradication.
In this study a new foreign breed of cherry tomatillo was used as the vector of producing transgenic plant vaccine. Through the research of transforming HBsAg gene into tomatillo, a high efficient transformation system of tomatillo was produced, and the recombinant protein from transgenic plants possessed good immunogenicity. The work demonstrated the feasibility of expressing the oral immunogenic rHBsAg in transgenic tomatillo, and provided some theoretic and experimental directions for the production oral vaccines using transgenic tomatillo in large scale.
Transgenic plants expressing recombinant antigens have been developed successfully since it was first described by Mason et al[15,22]. A corollary of this research is the goal of developing subunit vaccines that are produced in edible plant products, such that the plant-derived vaccine can be ingested directly without prior purification or processing. It appears to be a very promising alternative to other methods for expressing recombinant protein. Nevertheless, a number of questions and challenges still remain to be solved. And its main disadvantage is low yields of antigen expressed. To our knowledge, in most cases at present expression levels of vaccine proteins in transgenic plants are lower than 0.1%. We encountered the same problem in the study. Although plant-derived rHBsAg had good immunogenicity, the maximal level of rHBsAg in transgenic tomatillos was inadequate for use practically.
Bioencapsulation of the recombinant antigen within plant cells may protect it from degradation by gastrointestinal proteinases and allow important epitopes to be recognized. It may result in greater stability of the antigen in the stomach, effectively increasing the amount of antigen available for uptake in the gut. However, foreign antigens expressed in edible plant tissues for use as oral vaccines also will consist of impure mixtures with numerous other plant proteins. The stability of such a protein in the gut is unknown yet, but high protein levels and localization in the cell wall should ensure that at least some of the antigen will be delivered to the intestine. To address these possibilities, we need edible plant tissues that produce higher levels of rHBsAg, and thus our further studies will focus on increasing the accumulation of rHBsAg in transgenic tomatillos.
Over the past decade increasing endeavors have been directed towards how to augment expression levels of foreign genes in transgenic plants. Some modifications of expression vectors have been successfully assayed to have the function to lead to increased levels of expression of the recombinant genes, including the use of stronger promoters[15], plant-optimized synthetic genes[8], plant-derived leader sequences, signal peptides which can target the protein for retention in intercellular compartments[7,10,27], the fruit-specific expressed promoter[28,29], and the chloroplast expression system[30] etc.
Although this study was not yet complete, we could demonstrate that plant-derived rHBsAg was active as an oral immunogen and cherry tomatillo might be a potential source of vaccines for direct application. Transgenic tomatillos could induce specific immune response depending on the route of administration and immune status of the animal. In our hands, oral immunization solely by feeding transgenic tissues was not effective. Activation of the immune system by a primary immunization via a parenteral route followed by booster via the oral route resulted in significant antibody responses. The results showed that the administration of edible vaccines in primed instead of naive subjects revealed a more sensitive test system and higher probability of success. The mechanisms underlying the increased responsiveness of parenterally primed animals to orally delivered antigens are not clear yet. Further research is required to optimize this approach and to identify the underlying mechanisms. Combinations of parenteral and oral delivery may eventually prove to be most efficient, and in this regard, the present study provides a platform for ongoing and future investigations.
In conclusion, more and more attentions have been paid to the study on oral vaccine using transgenic plants in the recent years, and many great achievements have been attained in this field. However, there is a long way to go before practical use of oral vaccine. Many problems still need to be solved, such as how to improve the accumulation of target protein in edible tissues of transgenic plant, how to enlarge the production scale of plant oral vaccine, how to properly determine the suitable delivery method and dosage of plant oral vaccine.
Edited by Ren SY
| 1. | Wang SP, Xu DZ, Yan YP, Shi MY, Li RL, Zhang JX, Bai GZ, Ma JX. Hepatitis B virus infection status in the PBMC of newborns of HBsAg positive mothers.. World J Gastroenterol. 2000;6:58. |
| 2. | Daniell H, Streatfield SJ, Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci. 2001;6:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 389] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Walmsley AM, Arntzen CJ. Plants for delivery of edible vaccines. Curr Opin Biotechnol. 2000;11:126-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Koprowski H, Yusibov V. The green revolution: plants as heterologous expression vectors. Vaccine. 2001;19:2735-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Liu DH. Plant as a system for production of pharmaceutical proteins. Shengwu Jishu Tongbao. 1999;4:1-5. |
| 6. | Tacket CO, Reid RH, Boedeker EC, Losonsky G, Nataro JP, Bhagat H, Edelman R. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine. 1994;12:1270-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Haq TA, Mason HS, Clements JD, Arntzen CJ. Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science. 1995;268:714-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 362] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Mason HS, Haq TA, Clements JD, Arntzen CJ. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine. 1998;16:1336-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 209] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Tacket CO, Mason HS, Losonsky G, Clements JD, Levine MM, Arntzen CJ. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat Med. 1998;4:607-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 336] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 10. | Lauterslager TG, Florack DE, van der Wal TJ, Molthoff JW, Langeveld JP, Bosch D, Boersma WJ, Hilgers LA. Oral immunisation of naive and primed animals with transgenic potato tubers expressing LT-B. Vaccine. 2001;19:2749-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Streatfield SJ, Jilka JM, Hood EE, Turner DD, Bailey MR, Mayor JM, Woodard SL, Beifuss KK, Horn ME, Delaney DE. Plant-based vaccines: unique advantages. Vaccine. 2001;19:2742-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Chikwamba R, Cunnick J, Hathaway D, McMurray J, Mason H, Wang K. A functional antigen in a practical crop: LT-B producing maize protects mice against Escherichia coli heat labile enterotoxin (LT) and cholera toxin (CT). Transgenic Res. 2002;11:479-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Arakawa T, Chong DK, Langridge WH. Efficacy of a food plant-based oral cholera toxin B subunit vaccine. Nat Biotechnol. 1998;16:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 200] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Daniell H, Lee SB, Panchal T, Wiebe PO. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J Mol Biol. 2001;311:1001-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 257] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Mason HS, Lam DM, Arntzen CJ. Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci USA. 1992;89:11745-11749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 385] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Richter LJ, Thanavala Y, Arntzen CJ, Mason HS. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol. 2000;18:1167-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 238] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Kong Q, Richter L, Yang YF, Arntzen CJ, Mason HS, Thanavala Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc Natl Acad Sci USA. 2001;98:11539-11544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Smith ML, Mason HS, Shuler ML. Hepatitis B surface antigen (HBsAg) expression in plant cell culture: Kinetics of antigen accumulation in batch culture and its intracellular form. Biotechnol Bioeng. 2002;80:812-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Zhao CH, Wang R, Zhao CS, Wang GL, Tian P. Expression of human hepatitis B virus surface antigen gene with and without preS in transgenic tomato. Nongye Shengwu Jishu Xuebao. 2000;8:85-88. |
| 20. | Chen HY, Zhang J, Gao Y, Du HL, Ma Y, Zheng WZ, Xia NS. Transforming HBsAg into peanut and detection of its immunogenicity. Shengwu Jishu Tongxun. 2002;13:245-250. |
| 21. | Ma Y, Lin SQ, Gao Y, Zhang J, Lu LX, Xia NS. Transformation of HBsAg (hepatitis B virus surface antigen) into tomato plants. Fu jian Nonglin Daxue Xuebao(Ziran Kexueban). 2002;31:223-227. |
| 22. | Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci USA. 1996;93:5335-5340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 323] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ. Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis. 2000;182:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 267] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | McGarvey PB, Hammond J, Dienelt MM, Hooper DC, Fu ZF, Dietzschold B, Koprowski H, Michaels FH. Expression of the rabies virus glycoprotein in transgenic tomatoes. Biotechnology (N Y). 1995;13:1484-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Kapusta J, Modelska A, Figlerowicz M, Pniewski T, Letellier M, Lisowa O, Yusibov V, Koprowski H, Plucienniczak A, Legocki AB. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 1999;13:1796-1799. [PubMed] |
| 26. | Wang GL, Fang HJ. Principle and technology of plant genetic engineering, 1st ed, Beijing:. Science Press. 1998;. |
| 27. | Tackaberry ES, Dudani AK, Prior F, Tocchi M, Sardana R, Altosaar I, Ganz PR. Development of biopharmaceuticals in plant expression systems: cloning, expression and immunological re-activity of human cytomegalovirus glycoprotein B (UL55) in seeds of transgenic tobacco. Vaccine. 1999;17:3020-3029. |
| 28. | Clendennen SK, López-Gómez R, Gómez-Lim M, Arntzen CJ, May GD. The abundant 31-kilodalton banana pulp protein is homologous to class-III acidic chitinases. Phytochemistry. 1998;47:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Sandhu JS, Krasnyanski SF, Domier LL, Korban SS, Osadjan MD, Buetow DE. Oral immunization of mice with transgenic tomato fruit expressing respiratory syncytial virus-F protein induces a systemic immune response. Transgenic Res. 2000;9:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | De Cosa B, Moar W, Lee SB, Miller M, Daniell H. Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat Biotechnol. 2001;19:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 319] [Article Influence: 13.3] [Reference Citation Analysis (0)] |