Published online May 15, 2003. doi: 10.3748/wjg.v9.i5.910
Revised: October 5, 2002
Accepted: October 29, 2002
Published online: May 15, 2003
AIM: To investigate the expression of TFF2 and Helicobacter pylori infection in carcinogenesis of gastric mucosa.
METHODS: The expression of TFF2 was immunohistochemically analyzed in paraffin-embedded samples from 119 patients with endoscopic biopsy and subtotal gastrectomy specimens of gastric mucosal lesions, including 16 cases of chronic superficial gastritis (CSG), 20 chronic atrophic gastritis (CAG), 35 intestinal metaplasia (IM), 23 gastric epithelial dysplasia (GED) and 25 gastric carcinoma (CA), and Helicobacter pylori infection was detected by Warthin-Starry staining.
RESULTS: 1: TFF2 was located in the cytoplasm of gastric mucous neck cell. The expression of TFF2 was 100%, 100%, 0, 56.5% and 0 in CSGs, CAGs, IMs, GEDs and CAs, respectively. 2: The value of TFF2 positive cell density in CSG with Helicobacter pylori infection was higher than that without Helicobacter pylori infection. (52.89 ± 7.27vs 46.49 ± 13.04, P > 0.05); But the value of TFF2 positive cell density in CAG and GED with Helicobacter pylori infection was significantly lower than that without Helicobacter pylori infection (18.17 ± 4.09vs 37.93 ± 13.80, P < 0.01 and 14.44 ± 9.32vs 24.84 ± 10.22, P < 0.05).
CONCLUSION: Increase of TFF2 expression in CSG is perhaps associated with the protective mechanism after gastric mucosal injury. Decrease of TFF2 expression in CAG possibly attributes to the decrease in the number of gastric gland cell expressing TFF2. Re-expression of TFF2 in gastric epithelial dysplasia implies that TFF2 possibly contributes to the initiation of gastric carcinoma. The effect of Helicobacter pylori on the expression of TFF2 depends on the status of gastric mucosa.
-
Citation: Hu GY, Yu BP, Dong WG, Li MQ, Yu JP, Luo HS, Rang ZX. Expression of TFF2 and
Helicobacter pylori infection in carcinogenesis of gastric mucosa. World J Gastroenterol 2003; 9(5): 910-914 - URL: https://www.wjgnet.com/1007-9327/full/v9/i5/910.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i5.910
Trefoil factor family 2 (TFF2), also known as spasmolytic polypeptide (SP), is one of three known mammalian trefoil peptides. Trefoil peptides are small (7-12 kDa) proteaseresistant proteins secreted by the gastrointestinal mucosa in a lineage-specific manner. While expressed and secreted preferentially by gastric mucous neck cell[1,2], TFF2 is upregulated in diverse pathological conditions of gastrointestinal tract, which involves regeneration and restitution of epithelial lineage during the epithelial cell injury, mucosal protection and healing of ulcer[3-5]. However, the relationship between TFF2 expression and gastric carcinoma is still not fully elucidated. In the present study, we evaluated the expression of TFF2 and Helicobacter pylori infection in a series of gastric mucosal lesions. The aim of this study was in two aspect: to characterize the expression pattern of TFF2 on carcinogenesis of gastric mucosa; and to study the relationship between the expression of TFF2 and Helicobacter pylori infection.
The gastric mucosal lesions of all 119 patients who had undergone surgical resection or endoscopic biopsy in the Renmin Hospital of Wuhan University from March 2001 to March 2002 were studied. There were 16 cases of chronic superficial gastritis (CSG), 20 chronic atrophic gastritis (CAG), 35 intestinal metaplasia (IM), 23 gastric epithelial dysplasia (GED) and 25 gastric carcinoma (CA). Tissue fragments were fixed in 10% formaldehyde and embedded in paraffin. Serial sections of 4 μm were stained with haematoxylin and eosin (HE), Warthin-Starry and by immunohistochemistry.
A modification of the streptavidin-peroxidase method was applied to immunohistochemistry and with 3, 3’-diaminobenzidine (DAB) as the chromogen (Ultrasensitive SP Kit, DAB, FuZhou Maixin Biotechnology Co). Sections was incubated for one hour at 37 °C with monoclonal antibody against human TFF2 (hSP, diluted 1:35, Novocastra Laboratories Ltd). All batches of staining included positive control. Negative control was performed by replacing the primary mAbs with Tris buffer solution (TBS). The value of positive cell density was determined by image analysis system of HPIAS2000.
Histological examination with Warthin-Starry staining technique was used for Helicobacter pylori infection diagnosis in all cases, and was identified as positive brown-black or black staining of Helicobacter pylori.
Immunoreactivity were scored according to the presence of immunoreactive cells: -, none or rate positive cells (< 5%); +, 5%-25%; ++, 25%-75%; +++, > 75%.
All results are expressed as means plus or minus SD unless otherwise stated, and analyzed by software of SPSS 10.00. The statistical significance of difference was evaluated with the Student’s t test. A P value of less than 0.05 was considered statistically significant.
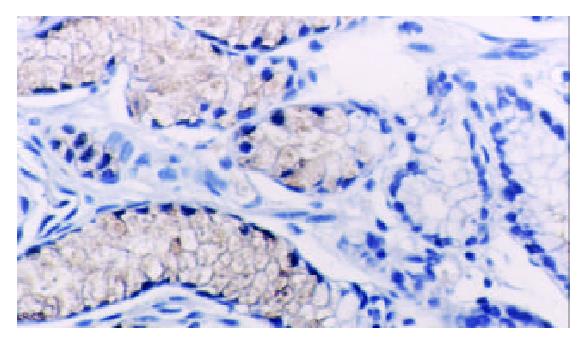
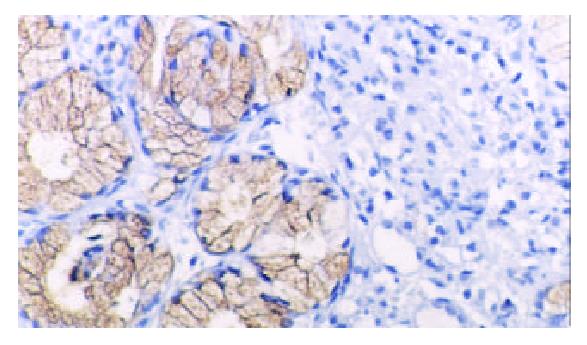
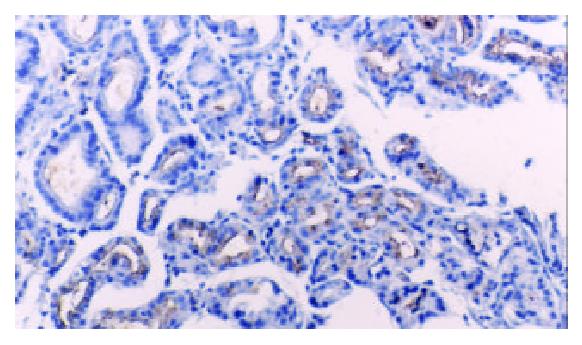
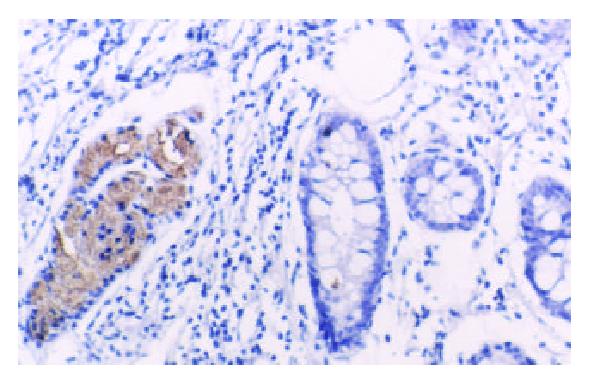
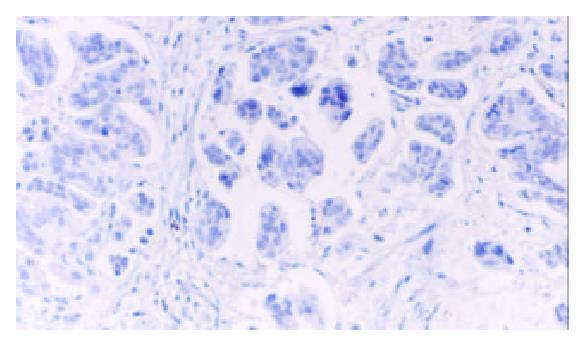
TFF2 protein was located in the cytoplasm of gastric epithelial cells and gastric gland mucous neck cells by immunohistochemistry, and positive cells were stained brown-yellow. TFF2 protein was expressed in all the chronic superficial gastritis and chronic atrophic gastritis (Figure 1, Figure 2), but the scoring of TFF2 staining was higher in chronic superficial gastritis than in that chronic atrophic gastritis. The expression of TFF2 was partly detected (56.5%) in the gastric epithelial dysplasia (Figure 3). There was no expression of TFF2 in the intestinal metaplasia and gastric carcinoma. (Figure 4, Figure 5) (Table 1).
| Classification | Cases | TFF2 | Rates of positivity | |||
| - | + | ++ | +++ | |||
| CSG | 16 | 0 | 1 | 5 | 10 | 100% |
| CAG | 20 | 0 | 5 | 11 | 4 | 100% |
| IM | 35 | 35 | 0 | 0 | 0 | 0 |
| GED | 23 | 10 | 9 | 2 | 2 | 56.5% |
| CA | 25 | 25 | 0 | 0 | 0 | 0 |
The positive rate of Helicobacter pylori was 53.8% (64/119) in our cases. The value of TFF2 positive cell density in chronic superficial gastritis with Helicobacter pylori infection was higher than that without Helicobacter pylori infection (52.89 ± 7.27 vs 46.49 ± 13.04, P > 0.05), but the difference was not statistically significant; While the value of that in chronic atrophic gastritis and dysplasia with Helicobacter pylori infection was significantly lower than that without Helicobacter pylori infection (18.17 ± 4.09 vs 37.93 ± 13.80, P < 0.01 and 14.44 ± 9.32 vs 24.84 ± 10.22, P < 0.05), and the difference was statistically significant (Table 2).
| Classification | Cases | TFF2 | t test |
| CSG | |||
| H. pylori (+) | 8 | 52.89 ± 7.27 | P > 0.05 |
| H. pylori (-) | 8 | 46.49 ± 13.04 | |
| CAG | |||
| H. pylori (+) | 12 | 18.17 ± 4.09 | P < 0.01 |
| H. pylori (-) | 8 | 37.93 ± 13.80 | |
| IM | |||
| H. pylori (+) | 16 | 0 | |
| H. pylori (-) | 19 | ||
| GED | |||
| H. pylori (+) | 12 | 14.44 ± 9.32 | P < 0.05 |
| H. pylori (-) | 11 | 24.84 ± 10.22 | |
| CA | |||
| H. pylori (+) | 15 | 0 | |
| H. pylori (-) | 9 |
We undertook the present study in order to characterize the pattern of TFF2 protein expression in carcinogenesis of gastric mucosa. In conformity with data in the literature[2], immunohistochemical expression of TFF2 was not observed at the surface epithelium, although expression of the corresponding mRNA was previously detected by in situ hybridization[2]. We observed, as did others, the expression of TFF2[2,6] in the mucous cells of the antrum, the chief cells of the body and neck zone cells.
In this study, we have clarified the expression pattern of TFF2 protein in carcinogenesis of gastric mucosa. In chronic superficial gastritis and atrophic gastritis, the high expression of TFF2 was observed in all cases. As a cytoprotective factor, TFF2 protein was induced as a result of gastric mucosal injury. Our results confirmed an important cytoprotective and restitutive role for TFF2. The mechanism of protective and healing effect of TFF2 on the gastric mucosa is still not fully elucidated. In vitro, TFF2 stimulated cell migration[7]. It has recently been shown that hTFF decreased proton permeation through interacting with mucus in vivo and in vitro[8], and oral TFF2 binds to the mucus layer of the stomach[9], which accelerates the gastric ulcer healing in rat. Further, we also discovered that the scores of TFF2 staining were higher in chronic superficial gastritis than that in chronic atrophic gastritis, which might attribute to the decrease in the number of mucous neck cell expressing TFF2 in chronic atrophic gastritis. There was no expression of TFF2 protein in intestinal metaplasia and gastric carcinoma, but TFF2 protein was observed in surrounding tissues of intestinal metaplasia and gastric carcinoma, which suggested the expression of TFF2 could be associated with the phenotype of gastric epithelial cell differentiation. Although it had been reported by Machado et al[10], the expression of TFF2 was observed in 10 (10.4%) of 96 cases of gastric carcinomas.
Furthermore, we also found that there were 13 out of 20 cases with re-expression of TFF2 in gastric epithelial dysplasia. It was well known that dysplasia is a precancerous lesion of stomach, and TFF2 could contribute to the initiation of gastric carcinoma. In 1999, Schemidt et al[11] found the SPEM (SP-expressing metaplasia) lineage was detected in 91% of gastric carcinoma, typically located in mucosa adjacent to the carcinoma or areas of dysphasia. And others studies[12] also discovered that SPEM was identified in 88% of the surrounding mucosa in the remnant cancers, as well as 61% of the follow-up biopsies. In the malignant resections, 67% of the surface dysplasia displayed SP positive cells. All findings implicated a strong association of the SPEM lineage with gastric carcinoma. At present, no confirmed relationship could be found between TFF2 and carcinoma[11-19]. However, Farrell et al[20] generated a model of TFF2-deficient mice, and suggested a physiologic role of TFF2 in promoting mucosal healing through the stimulation of proliferation and downregulation of gastric acid secretion. In other words, increased TFF2 expression and secretion could contribute directly to gastric cancer risk not only through stimulation of proliferation, but also through inhibition of acid secretion. Meanwhile, our results and others[11] showed there was loss of TFF2 in all gastric carcinoma cases. Therefore, it is impossible that TFF2 expression plays an important role in the progression of gastric carcinoma. Whether TFF2 can directly or indirectly contribute to carcinogenesis of gastric mucosa will be required for further studies.
Gastric carcinoma is the most common tumor in gastrointestinal tract[21-32], more and more studies suggested that Helicobacter pylori infection was significantly associated with gastric carcinoma and was a high risk factor for gastric carcinoma[33-52]. It has been reported that an increase in the number of mucous neck cells expressing TFF2 has been observed in both Helicobacter infected human patients[12] and Helicobacter-infected mice with both preneoplastic[53] and neoplastic[54] changes of the gastric mucosa. In all of these findings, it was likely that Helicobacter pylori infection would contribute to the expression of TFF2 by promoting the proliferation of gastric mucosal cell, which would be a mechanism of Helicobacter pylori contributing to carcinogenesis of gastric mucosa. Then, we examined retrospectively the Helicobacter pylori infection of all cases by Warthin-Starry staining, and found that the value of TFF2 positive cell density was higher in chronic superficial gastritis with Helicobacter pylori infection than that without, which suggested that TFF2 was induced in the early stage of Helicobacter pylori infection. Nevertheless, its value was significantly lower in chronic atrophic gastritis and dysplasia with Helicobacter pylori infection than that without. We inferred that low expression of TFF2 was associated with a decrease in the number of gastric mucosal cell as result of Helicobacter pylori infection. As a result, the effect of Helicobacter pylori on the expression of TFF2 could depend on the status of gastric mucosa.
In conclusion, early-stage or short-term upregulation of TFF2 appears to be helpful in healing of gastric mucosa, but prolonged up regulation may in fact contribute to carcinogenesis, Further studies will be needed to define the role of TFF2 in Helicobacter pylori-associated chronic gastritis and gastric carcinoma.
Edited by Zhang JZ
| 1. | Tomasetto C, Rio MC, Gautier C, Wolf C, Hareuveni M, Chambon P, Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990;9:407-414. [PubMed] |
| 2. | Hanby AM, Poulsom R, Singh S, Elia G, Jeffery RE, Wright NA. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993;105:1110-1116. [PubMed] |
| 3. | McKenzie C, Thim L, Parsons ME. Topical and intravenous administration of trefoil factors protect the gastric mucosa from ethanol-induced injury in the rat. Aliment Pharmacol Ther. 2000;14:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Tran CP, Cook GA, Yeomans ND, Thim L, Giraud AS. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut. 1999;44:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Cook GA, Familari M, Thim L, Giraud AS. The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid tissues and participate in the immune response. FEBS Lett. 1999;456:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Playford RJ, Marchbank T, Chinery R, Evison R, Pignatelli M, Boulton RA, Thim L, Hanby AM. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995;108:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 323] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Tanaka S, Podolsky DK, Engel E, Guth PH, Kaunitz JD. Human spasmolytic polypeptide decreases proton permeation through gastric mucus in vivo and in vitro. Am J Physiol. 1997;272:G1473-G1480. [PubMed] |
| 9. | Poulsen SS, Thulesen J, Christensen L, Nexo E, Thim L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. Gut. 1999;45:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Machado JC, Nogueira AM, Carneiro F, Reis CA, Sobrinho-Simões M. Gastric carcinoma exhibits distinct types of cell differentiation: an immunohistochemical study of trefoil peptides (TFF1 and TFF2) and mucins (MUC1, MUC2, MUC5AC, and MUC6). J Pathol. 2000;190:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639-646. [PubMed] |
| 12. | Yamaguchi H, Goldenring JR, Kaminishi M, Lee JR. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig Dis Sci. 2002;47:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, Sauvanet A, Lemoine NR. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol. 2002;160:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 179] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Azarschab P, Al-Azzeh E, Kornberger W, Gött P. Aspirin promotes TFF2 gene activation in human gastric cancer cell lines. FEBS Lett. 2001;488:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Ohshio G, Suwa H, Kawaguchi Y, Imamura M, Yamaoka Y, Yamabe H, Matsumoto M, Yoshioka H, Hashimoto Y, Takeda H. Differential expression of human spasmolytic polypeptide (trefoil factor family-2) in pancreatic carcinomas, ampullary carcinomas, and mucin-producing tumors of the pancreas. Dig Dis Sci. 2000;45:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Nogueira AM, Machado JC, Carneiro F, Reis CA, Gött P, Sobrinho-Simões M. Patterns of expression of trefoil peptides and mucins in gastric polyps with and without malignant transformation. J Pathol. 1999;187:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Labouvie C, Machado JC, Carneiro F, Sarbia M, Vieth M, Porschen R, Seitz G, Blin N. Differential expression of mucins and trefoil peptides in native epithelium, Barrett's metaplasia and squamous cell carcinoma of the oesophagus. J Cancer Res Clin Oncol. 1999;125:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Efstathiou JA, Liu D, Wheeler JM, Kim HC, Beck NE, Ilyas M, Karayiannakis AJ, Mortensen NJ, Kmiot W, Playford RJ. Mutated epithelial cadherin is associated with increased tumorigenicity and loss of adhesion and of responsiveness to the motogenic trefoil factor 2 in colon carcinoma cells. Proc Natl Acad Sci USA. 1999;96:2316-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | dos Santos Silva E, Kayademir T, Regateiro F, Machado JC, Savas S, Dobosz T, Blin N, Gött P. Variable distribution of TFF2 (Spasmolysin) alleles in Europeans does not indicate predisposition to gastric cancer. Hum Hered. 1999;49:45-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, Podolsky DK, Wang TC. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Cai L, Yu SZ, Zhan ZF. Cytochrome P450 2E1 genetic polymorphism and gastric cancer in Changle, Fujian Province. World J Gastroenterol. 2001;7:792-795. [PubMed] |
| 22. | Xiao HB, Cao WX, Yin HR, Lin YZ, Ye SH. Influence of L-methionine-deprived total parenteral nutrition with 5-fluorouracil on gastric cancer and host metabolism. World J Gastroenterol. 2001;7:698-701. [PubMed] |
| 23. | He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deletion [was deleton] and mutation of p16 gene in human gastric cancer. World J Gastroenterol. 2001;7:515-521. [PubMed] |
| 24. | Cai L, Yu SZ, Zhang ZF. Glutathione S-transferases M1, T1 genotypes and the risk of gastric cancer: a case-control study. World J Gastroenterol. 2001;7:506-509. [PubMed] |
| 25. | Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500-505. [PubMed] |
| 26. | Niu WX, Qin XY, Liu H, Wang CP. Clinicopathological analysis of patients with gastric cancer in 1200 cases. World J Gastroenterol. 2001;7:281-284. [PubMed] |
| 27. | Xin Y, Li XL, Wang YP, Zhang SM, Zheng HC, Wu DY, Zhang YC. Relationship between phenotypes of cell-function differentiation and pathobiological behavior of gastric carcinomas. World J Gastroenterol. 2001;7:53-59. [PubMed] |
| 28. | Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848-854. [PubMed] |
| 29. | Cai L, Yu SZ, Ye WM, Yi YN. Fish sauce and gastric cancer: an ecological study in Fujian Province,China. World J Gastroenterol. 2000;6:671-675. [PubMed] |
| 30. | Deng DJ. progress of gastric cancer etiology: N-nitrosamides 1999s. World J Gastroenterol. 2000;6:613-618. [PubMed] |
| 31. | Jiang BJ, Sun RX, Lin H, Gao YF. Study on the risk factors of lymphatic metastasis and the indications of less in vasive operations in early gastric cancer. World J Gastroenterol. 2000;6:553-556. [PubMed] |
| 32. | Wang ZN, Xu HM. Relationship between collagen IV expression and biological behavior of gastric cancer. World J Gastroenterol. 2000;6:438-439. [PubMed] |
| 33. | Lu SY, Pan XZ, Peng XW, Shi ZL, Lin L, Chen MH. Effect of Hp infection on gastric epithelial cell kinetics in stomach diseases. Shijie Huaren Xiaohua Zazhi. 2000;8:386-388. |
| 34. | Wang DX, Fang DC, Li W, Du QX, Liu WW. A study on relationship between infection of Helicobacter pylori and inactivation of antioncogenes in cancer and pre-cancerous lesion. Shijie Huaren Xiaohua Zazhi. 2001;9:984-987. |
| 35. | Guo CQ, Wang YP, Liu GY, Ma SW, Ding GY, Li JC. Study on Helicobacter pylori infection and -p53, c-erbB-2 gene expression in carcinogenesis of gastric mucosa. Shijie Huaren Xiaohua Zazhi. 1999;7:313-315. |
| 36. | He XX, Wang JML, Wu JL, Yuan SY, Ai L. Telomerase expression, Hp infection and gastric mucosal carcinogenesis. Shijie Huaren Xiaohua Zazhi. 2000;8:505-508. |
| 37. | Lu W, Chen LY, Gong HS. PCNA and c-erbB-2 expression in gastric mucosal intestinal metaplasia with Helicobacter pylori infection. Shijie Huaren Xiaohua Zazhi. 1999;7:111-113. |
| 38. | Yang Y, Deng CS, Yao XJ, Liu HY, Chen M. Effect of Helicobacter pylori on morphology and growth of gastric epithelial cells. Shijie HuarenXiaohua Zazhi. 2000;8:500-504. |
| 39. | Lu SY, Pan XZ, Peng XW, Shi ZL. Effect of Hp infection on gastric epithelial cell kinetics in stomach diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:760-762. |
| 40. | Liang HJ, Gao JH, Liu WW, Fang DC, Men RP. Longterm effects of concentrated Helicobacter pylori culture supernatant on gastric mucosa of rats. Shijie Huaren Xiaohua Zazhi. 1999;7:861-863. |
| 41. | Gao JH, Liang HJ, Liu WW, Fang DC, Wang ZH. Expression of C-myc gene protein and epidermal growth factor receptor in gastric mucosa pre and post Helicobacter pylori clearance. Shijie Huaren Xiaohua Zazhi. 1999;7:1018-1019. |
| 42. | Peng ZS, Liang ZC, Liu MC, Ouyang NT. Studies on gastric epithelial cell proliferation and apoptosis in Hp associated gastric ulcer. Shijie Huaren Xiaohua Zazhi. 1999;7:218-219. |
| 43. | Liu HF, Liu WW, Fang DC, Gao JH, Wang ZH. Apoptosis and proliferation induced by Helicobacter pylori and its association with p53 protein expression in gastric epithelial cells. Shijie Huaren Xiaohua Zazhi. 2001;9:1265-1268. |
| 44. | Liu HF, Liu WW, Fang DC, Yang SM, Zhao L. Gastric epithelial apoptosis induced by Helicobacter pylori and its relationship with Bax protein expression. Shijie Huaren Xiaohua Zazhi. 2000;8:860-862. |
| 45. | Maeda S, Yoshida H, Ogura K, Mitsuno Y, Hirata Y, Yamaji Y, Akanuma M, Shiratori Y, Omata M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119:97-108. [PubMed] |
| 46. | Ekström AM, Held M, Hansson LE, Engstrand L, Nyrén O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 47. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [PubMed] |
| 48. | Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779-782. [PubMed] |
| 49. | Yao YL, Xu B, Song YG, Zhang WD. Overexpression of cyclin E in Mongolian gerbil with Helicobacter pylori-induced gastric precancerosis. World J Gastroenterol. 2002;8:60-63. [PubMed] |
| 50. | Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248-253. [PubMed] |
| 51. | Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, Dite P, Läuter J, Labenz J, Leodolter A. Helicobacter pylori and gastric cancer: current status of the Austrain Czech German gastric cancer prevention trial (PRISMA Study). World J Gastroenterol. 2001;7:243-247. [PubMed] |
| 52. | Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374-376. [PubMed] |
| 53. | Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 445] [Article Influence: 17.8] [Reference Citation Analysis (0)] |