INTRODUCTION
Transport of protein in the form of small peptides (di/tripeptides) across the small intestinal wall is a major route of dietary protein absorption. The H+-coupled dipeptide transporter, PepT1, is known to be located in the intestine and the kidney, and plays an important role in the absorption of di/tripeptide; in addition, it mediates the intestinal absorption of β-lactam antibiotics, angiotensin-converting enzymeinhibitors, and other peptide-like drugs[1].
To investigate the properties of the human dipeptide transporter, the human intestinal cell line Caco-2 as an in vitro model was employed. The dipeptide transporters normally found in the small intestine are present in Caco-2 cells[2-4]. Also, Caco-2 cells spontaneously differentiate in culture to polar cells possessing microvilli and enterocytic properties[5]. Confluent monolayers form tight junctions between cells[5], and exhibit dome formation[6] and electrical properties of an epithelium[7]. These cells have been evaluated in detail as a model to study both transcellular and paracellular transport of nutrients and drugs in the gut[8-12].
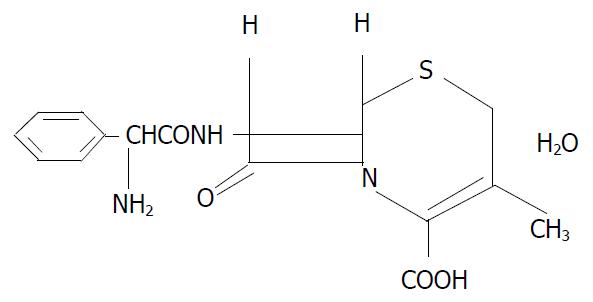
Previous studies have shown that some hormones metabolically regulate the expression of the intestinal dipeptide transporter[13,14]. For example, as the key hormone, insulin increases the membrane population of PepT1 by increasing its translocation from a preformed cytoplasmic pool[14]. However, little is known whether another important hormone, growth hormone (GH), also metabolically regulate the functions of PepT1. The present study was undertaken to evaluate the potential effects of recombinant human growth hormone (rhGH) for the study of the properties of the human PepT1. Especially, we observed the regulation effects of rhGH to PepT1 in Caco-2 cells which were normally cultured or anoxia/reoxygenation injuried. Because dipeptide and some β-lactam antibiotics (cephalexin, cefaclor etc.) share certain structural features such as a peptide bond with an α-amino group and a terminal carboxylic acid group (as illustrated in Figure 1 for cephalexin), it is not surprising that these compounds share a common transport mechanism. Unlike dipeptides, these orally absorbed antibiotics are not hydrolyzed by intestinal peptidases. Consequently, these kinds of drugs are ideal substrates to characterize the PepT1[15-17]. In this study, the uptake and transport of cephalexin were examined.
Figure 1 Structure of cephalexin used in this study.
MATERIALS AND METHODS
Materials
Cephalexin (Figure 1) was obtained from Nanjing No.2 Pharma.Co. Ltd. rhGH was purchased from Laboratoires Serono S.A. (Switzerland). All other chemicals were purchased from Sigma (St. Louis, MO), unless specified. Cell culture reagents were obtained from Gibco (Grand Island, NY) and culture supplies from Corning (Corning, NY) and Falcon (Lincoln Park, NJ).
Cell culture
The human adenocarcinoma cell line Caco-2 was obtained from American Tissue Culture Collection (ATCC, Rockville. MD, U.S.A). The cells were passaged as previously described[18]. Caco-2 cells were grown in Dulbecco’s Modified Egle’s Medium supplemented with 1% nonessential amino acids, 10% fetal calf serum (FCS), 1000 UL-1 penicillin, and 1 mg·L-1 streptomycin (Complete DMEM). For transport Studies, Caco-2 cells were seeded on the polycarbonate filter (0.4-µm pores, 4.71-cm2 growth area) in the Transwell Cell Culture Chamber System (Costar, Cambridge, MA) at a density of 600000 cells/filter. The cell monolayers were cultured with 1.5 and 2.6 ml of complete DMEM at the apical and the basolateral chamber, respectively. To assess the integrity of the monolayer, transepithelial electrical resistance (TEER) was monitored by measuring the transmembrane resistance. After subtracting intrinsic resistance (filter alone without cell monolayers) from the total resistance, TEER was corrected for surface area and expressed as Ω cm2. Transport studies were carried out with cell monolayers that were 21-25 d old. For uptake studies, cells were seeded in the collagen-coated multiwell dishes (24 pore) with the same cell density. All the medium were replaced every day when each experiment began. Monolayers were kept at 37 °C, 5% CO2, and 90% related humidity.
Transport studies
Before the transport experiments, the culture medium was replaced every day from both sides of the monolayers, 1.5 ml pH7.0 culture medium with 34 nM rhGH were added to apical chamber of Transwell, while 2.6 ml pH7.0 culture medium without rhGH were added to basolateral chamber. Then Caco-2 cells were cultured for 4 d. The controls were incubated with PH7.0 culture medium both in apical side and basolateral side. All the monolayers were washed during each experiment. The apical chamber were then filled with 1.5 ml solution containing 1 mM cephalexin. Transport was examined within the time range of 0, 5, 10, 30, 60, 90, and 120 min. At the end of the incubation period, the inserts of Transwell were removed and the transport samples were obtained from the basolateral chambers. The concentrations of cephalexin of the transport samples were determined by High Performance Liquid Chromatography (HPLC, LC-10AT SHIMADIU, Japan) using an isocratic solvent system described previously[19] and the OD value was monitored at 254 nm.
Uptake studies
Using the same way as the transport experiments, the Caco-2 cells cultured in the multiwell dishes (24 pore) were cultured for 4 d with 34 nM rhGH. After washing the monolayers, the transport medium with 1 mM cephalexin was added to the pores and uptake was measured at 0, 5, 10, 30, 60, 90, and 120 min. At the end of the incubation period, cell monolayers were washed with pH7.4 PBS to remove any extracellular cephalexin. Accumulated concentrations of cephalexin were determined by HPLC after the cells were lysed.
Anoxia-reoxygenation cells model
After Caco-2 cells were plated, media within the wells was reduced to a uniformly thin layer to reduce the diffusion distances for atmospheric gases, while maintaining cell ability. During culture, the monolayers were normally exposed to an atmosphere consisting of 95% air and 5% CO2. Anoxia was produced with an atmosphere of 95% N2 and 5% CO2 for 90 min, reoxygenation was produced by restoration of the 95% air, 5% CO2 for 30 min. After the period of reoxygenation, the volume of media was restored to the initial volume of 1.0 ml[20] (Figure 2).
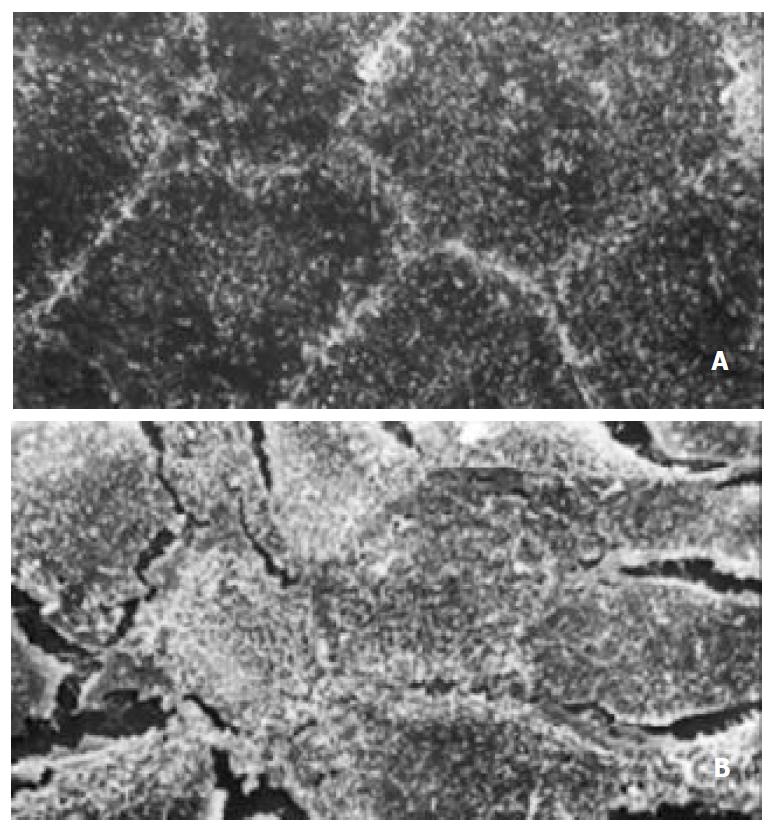
Figure 2 Appearance of scan electronic microscopy of Caco-2 cells.
(A) Tight junction formation of normal Caco-2 cells. × 1000; (B) Tight junction of A/R Caco-2 cells was destroyed partially. × 1000.
Protein determination
Cells were lysed for 20 min or ice in 2% Nonidet P-40, 0.2% SDS, 1 mM dithiothreitol (DTT) in PBS, and total cellular protein was determined using Dc Protein Assay Reagent (BioRad).
Concentration dependence of cephalexin transport
To examine the kinetics of cephalexin transport by rhGH-treated Caco-2 cells, the different concentrations of cephalexin range of 20 to 80 µg·ml-1 were used.
Northern blotting
Total RNAs were isolated from the Caco-2 cell fractions by extraction with acid guanidine thiocyanate-phenol-chloroform. Total RNAs were denatured by heating at 70 °C in 10 mmol·L-1 3-(N-morpholino) propanesulfonic acid (pH7.0) containing 5 mmol·L-1 sodium acetate, 1 mmol·L-1 ethylenedi-aminetetraacetic acid (EDTA), 2.2 mol·L-1 formaldehyde, and 50% (vol/vol) formamide for 5 minutes and subjected to electrophoresis in 1.2% agarose gel containing 2.2 mol·L-1 formaldehyde. Resolved RNA was transferred to hybridization and was performed in a solution that contains 50% formamide, 5x sodium chloride-sodium phosphate-EDTA, 2x Denhardt’s solution, and 1% sodium dodecyl sulfate (SDS). The membranes were exposed and analyzed by Fuji BAS-2000 system. The cDNA probe was prepared from human cDNA libraries. Sense 5’TTTGGATCCCGGAGTAAGGCATTTCCCAAG3’, Antisense 5’CCGAAGCTTTCAGGAAGGAGCCTGAGAATATGAG3’.
Statistical analysis
Data were expressed as mean ± SE. Differences between experimental groups were assessed by analysis of Variance. P values of < 0.05 were considered statistically significant.
RESULTS
Transepithelial cephalexin transport in Caco-2 cell with normal culture
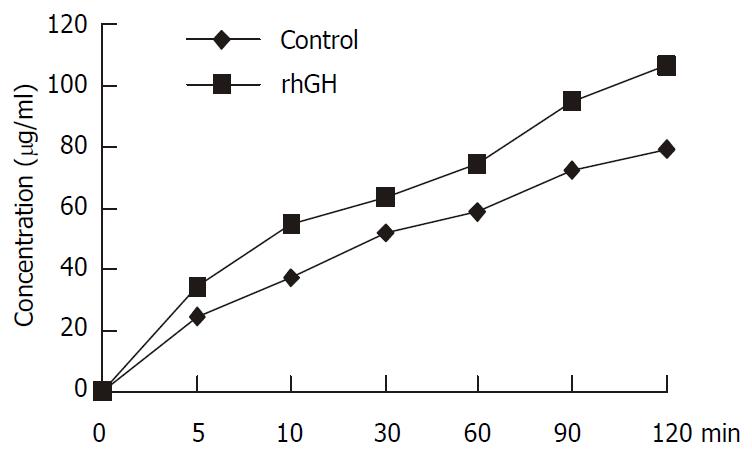
Caco-2 cells were seeded at a high density of 600000 cells/filter, the transport of 1 mM cephalexin across rhGH-treated Caco-2 monolayers, apical-to-basolateral, was examined at PH6.0 in the donor and pH7.4 in the receiver compartment. Integrity of the monolayers was monitored by TEER. The apical-to-basolateral transport of cephalexin was significantly increased compared with the controls (P = 0.0045) (Figure 3).
Figure 3 Transport of 1 mM cephalexin in Caco-2 cells.
Caco-2 cells were cultured with rhGH in apical side for 4 d. The controls were incubated with PH7.0 culture medium without rhGH both in apical side and basolateral side. All the mono-layers were washed in each experiment. The apical chambers were then filled with 1.5 ml solution containing 1 mM cephalexin. Transport was examined within the time range of 0, 5, 10, 30, 60, 90, and 120 min. At the end of the incubation period, the inserts of Transwell were removed and the trans-port samples were obtained from the basolateral chambers. The concentrations of cephalexin of the transport samples were determined by HPLC. The apical-to-basolateral transport of cephalexin was significantly increased compared with the con-trols (P = 0.0045).
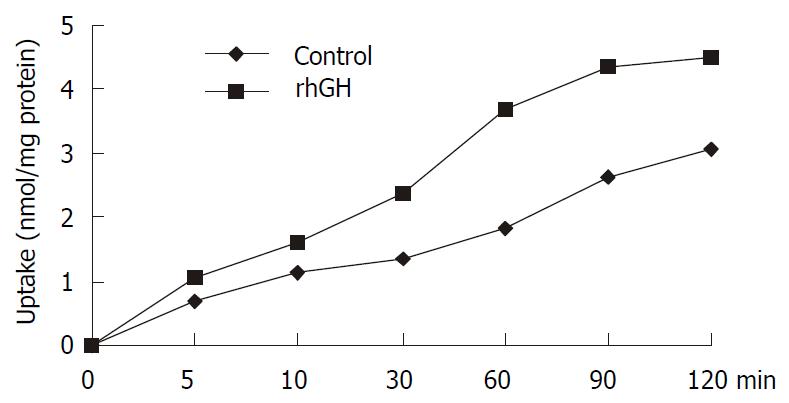
Uptake of cephalexin in Caco-2 cells with normal culture
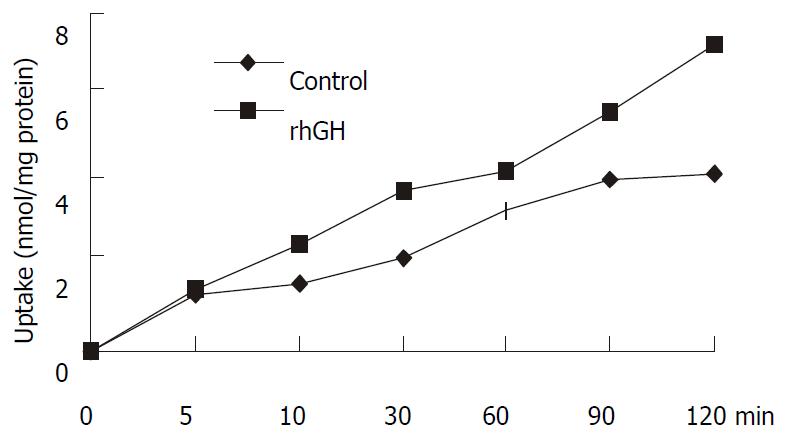
Uptake experiments with cephalexin were performed 6-8 d affter seeding and 18 h after media replacement. Cephalexin uptake was measured in the presence and absence of 34 nM of rhGH. The differences were significant (P = 0.0223), and the results were summarized in Figure 4.
Figure 4 Uptake of 1 mM cephalexin in Caco-2 cells.
Caco-2 cells cultured in the multiwell dishes (24 pore) were cultured for 4 d with 34 nM rhGH. After washing the monolayers, the transport medium with 1 mM cephalexin was added to the pores and uptake was measured at 0, 5, 10, 30, 60, 90, and 120 min. At the end of the incubation period, cell monolayers were washed with PH7.4 PBS to remove any extracellular cephalexin. Accumulated concentrations of cephalexin were determined by HPLC after the cells were lysed. The differences were significant (P = 0.0223).
Uptake of cephalexin in anoxia/reoxygenation Caco-2 cells model
Caco-2 monolayers were initially subjected to a 90-minute period of anoxia followed by a 30-minute period of reoxygenation. This was selected as optimal for the main body of the experiment on the basis of the results of our initial experiments. Consequently, the effect of the presence of rhGH on cephalexin uptake via the PepT1 of anoxia/reoxygenation Caco-2 cells were examined (Figure 5). The present studies indicated that uptake and transport of cephalexin by the normal and anoxia/reoxygenation Caco-2 cells were significantly increased under the influence of rhGH.
Figure 5 Uptake of 1 mM cephalexin in Caco-2 cells with an-oxia/reoxygenation injury.
Caco-2 monolayers were initially subjected to a 90-minute period of anoxia followed by a 30-minute period of reoxygenation. The effect of the presence of rhGH on cephalexin uptake via the PepT1 of anoxia/reoxygenation Caco-2 cells was examined. The differences were significant (P = 0.0116).
Northern blot analysis
Northern blots of Poly (A)+ RNA, prepared from anoxia/reoxygenation Caco-2 cells grown either under control conditions or in medium supplemented with 34 nM rhGH, were probed separately with 32P-labelled hPepT1 and β-actin cDNAs. The studies showed that the level of mRNA in rhGH-treated Caco-2 Cells was increased 1.3-fold compared with the controls.
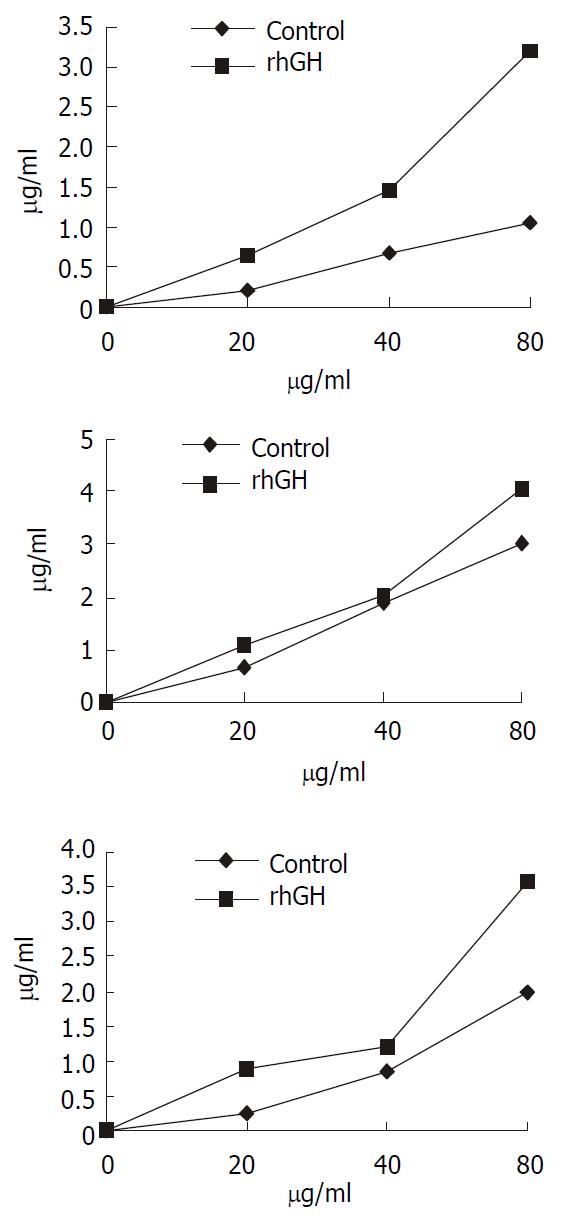
Concentration dependence of cephalexin transport
Transport experiments were performed across the rhGH-treated Caco-2 cells using the different concentrations of cephalexin range of 20 to 80 µg·ml-1. The results (Figure 6) indicated that the transport of cephalexin was increased both in rhGH-treated Caco-2 cells and Controls. However, the extent of upregulation of rhGH on the transport was greater than the controls followed the increase of the concentration of cephalexin.
Figure 6 Concentration-dependent transport of cephalexin.
The kinetics of cephalexin transport by rhGH-treated Caco-2 cells showed that the transport level of Caco-2 cells was in ac-cordance with the concentration of cephalexin. The concentra-tions of cephalexin range of 20 to 80 µg·ml-1 were used. Trans-port was determined at the different time-point (a, 15 min; b, 30 min; c, 45 min). The extent of upregulation of rhGH on the transport was greater than the controls followed the increase of the concentration of cephalexin.
DISCUSSION
The nutritional importance of amino acid uptake in the form of short-chain peptides (di/tripeptides) is well known[21]. The intraluminal products of protein digestion are predominantly small peptides[22]. A significant fraction of the dietary amino nitrogen is absorbed as intact dipeptide rather than free amino acids. Absorption of these small peptieds from the gastrointestinal (GI) tract of mammals occurs via the dipeptide transporter (PepT1). Previous studies have shown that the functions of intestine (including PepT1) were changed under the influence of many factors[23,24]. PepT1 seems to play important roles in nutritional and pharmacological therapies; for example, it has allowed the use of small peptide as a source of nitrogen for enteral feeding and the use of oral route for delivery of peptididomimetic drugs such as β-lactam antibiotics. PepT1 was located at the brush-border membranes of the absorptive epithelial cells along the small intestine but absent in crypt and goblet cells[25,26].
To examine the functions of PepT1, we used Caco-2 cells (the human adenocarcinoma cell line) as a unique in vitro intestinal model[27]. One clear advantage of the Caco-2 cells is that the cells stay viable throughout the transport studies. Also, a monolayer of cells may be grown on a porous support to represent an intact epithelium. Some β-lactam anibiotics (cephalexin) and dipeptied share certain structural features, and they are generally in the D-form, which allows them to escape hydrolysis by cytoplasmic peptidases. So these compounds share a common transport mechanism[28]. In this study, we employed cephalexin as an ideal substrate.
The previous studies have shown that the insulin stimulates dipeptide transport by increasing membrane insertion of PepT1 from a preformed cytoplasmic Pool[14], and choleratoxin decreases dipeptide transport by inhibiting the activity of PepT1 through an increase in the intracellular concentration of adenosine 3’, 5;-cyclic monophosphate[21]. It remains unclear, however, whether another key hormone, human growth hormone (hGH), also shows some significant importance. Strong evidence has demonstrated that growth hormone (GH) is an important growth factor for intestine[28]. Complete GH depletion due to hypophysectomy caused pronounced hypoplasia of small intestinal mucosa with decreased villus height and reduced crypt cell proliferation[29]. Simple replacement of GH can restore mucosal proliferative activity[30]. rhGH promotes normal growth and development in the body by changing chemical activity in cells. It activates protein production in muscle cells and the release of energy from fats. rhGH significantly improves the anabolism in parenterally fed[31]. It is typically used to stimulate growth in children with hormone deficiency, or to treat people with severe illness, burns or sepsis where destruction of human tissue and muscle occurs[32-34]. Many of the effects of human growth hormone are brought about by the insulin-like growth factor 1 (IGF-1), which the growth hormone stimulates. IGF-1 plays an important part in growth-promoting effects of rhGH[32]. The present studies showed that the uptake and transport of cephalexin in Caco-2 cells were greatly increased by using of rhGH. In addition, a specific injured cell model, anoxia/reoxygenation Caco-2 cells model, was established in our present experiment. The uptake of cephalexin in the injured Caco-2 cells was markedly decreased while the cephalexin uptake was significantly increased in the injured Caco-2 cells with treatment of rhGH. These results indicate that the functions of PepT1 in Caco-2 cells were upregulated by rhGH. The investigation of concentration-dependent transport of cephalexin in Caco-2 cells showed that the transport was increased both in rhGH-treated Caco-2 cells and controls. However, the upregulating extent of rhGH on the transport of cephalexin was greater than controls following the increase of the concentration of cephalexin. Northern blot analysis showed that the level of PepT1 mRNA of injured Caco-2 cells with rhGH treatment were increased. This result also provides novel information about the mechanism of regulation action of rhGH. It has suggested that the alteration in the gene expression may be a mechanism of regulation of PepT1.
Use of this experimental design leads to the following three questions. What is the detail mechanism of upregulating the functions of PepT1 by rhGH? How does the rhGH receptor distribute in Caco-2 cells? How does the rhGH receptor change in anoxia/reoxygenation Caco-2 cells? Clearly, the present study needs to be followed by further studies on physiology and biology of hormonal regulation of PepT1.