Published online Feb 15, 2003. doi: 10.3748/wjg.v9.i2.338
Revised: August 24, 2002
Accepted: September 2, 2002
Published online: February 15, 2003
AIM: To determine whether Platelet activating factor (PAF) has a regulation role in the expression of adhesion molecules and accumulation of neutrophils in a murine model of acute pancreatitis.
METHODS: One hundred twenty-eight Kunming mice were divided into four groups. Group 1 received 0.1 mL saline s.c. every hour for three hours (sham). Group 2 received cerulein (50 μg/kg dose s.c.) every hour for three hours. Group 3 received AP and additional challenge of PAF (50 mg/kg in absolute ethanol) (AP/PAF). Group 4 received AP, plus therapeutic treatment with GAB (25 mg dose i.p.) immediately after the first challenge of cerulein (AP/GAB). Animals were sacrificed at 12 h after the first challenge of saline or cerulein. Adhesion molecules of pancreas were semi-quantified by SP methods. Standard assays were performed for serum amylase and myeloperoxidase activity (MPO) of pancreas. Histology of pancreas was scored in a blind manner. Water content of pancreas was also measured at the same time.
RESULTS: Control pancreata showed negligible adhesion molecule expression and neutrophil accumulation. There were evident adhesion molecules expression and neutrophil accumulation in AP and AP/PAF compared with sham (P < 0.05). AP/GAB had a lower level of adhesion molecules, neutrophils, and water content versus AP and AP/PAF (P < 0.05). Histology showed a trend toward improvement in AP/GAB, but did not reach statistical significance.
CONCLUSION: PAF can induce the expression of adhesion molecules that mediate neutrophil accumulation. The PAF antagonist reduces the expression of adhesion molecules and the severity of inflammation when given immediately after the induction of mild AP in mice. These results suggest that PAF antagonism may be useful in the treatment of mild pancreatitis after its clinical onset.
- Citation: Zhao H, Chen JW, Zhou YK, Zhou XF, Li PY. Influence of platelet activating factor on expression of adhesion molecules in experimental pancreatitis. World J Gastroenterol 2003; 9(2): 338-341
- URL: https://www.wjgnet.com/1007-9327/full/v9/i2/338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i2.338
Acute pancreatitis (AP) was characterized as local and systemic inflammatory reactions. Adhesion molecules play a pivotal role in neutrophil immigration and accumulation[1]. Platelet activating factor (PAF) is a biologically active phospholipid which is thought to function as one of the proximal mediators in the inflammatory cascade of AP. However, the relationship between PAF and the expression of adhesion molecules in AP remains largely unknown, although Blackstone MO has ever speculated[2].
The present study was conducted to determine whether PAF has a regulatory role in the expression of adhesion molecules and the accumulation of neutrophils. To examine this effect, a murine model of edematous pancreatitis was established by overdose administration of cerulein, as described by Tani et al[3]. PAF and its antagonist (GAB) were also used in this experiment.
Cerulein and PAF were purchased from Sigma Chemical Co. (St.Louis, Missouri). GAB (Ginkgolide AB) was a generous gift from associate professor Di-Qing Zhang in Guizhou University. MPO assay kit was purchased from Nanjing Jiancheng Bioengineering Co. Ltd. China. Rabbit anti-rat E-selectin polyclonal antibody and Rabbit anti-rat ICAM-1 polyclonal antibody were purchased from Boster Bioengineering Co. Ltd. China. SP assay kit was purchased from Maixin Bioengineering Co. Ltd. China. Cerulein was dissolved in normal saline at the concentration of 10 μg/mL. PAF was first dissolved in absolute ethanol, and then diluted with normal saline when it was to be used. GAB was dissolved in 30% Dimethyl Sulfoxide (DMSO).
128 six-week-old Kunming mice, weighing 34-36 g each, were purchased from the experimental animal center of Wuhan University. The animals were randomly divided into 4 groups (described in methods) and fed standard laboratory chow. All animals were allowed to acclimatize for a minimum of 1 wk prior to experimentation. Before the day of the experiment, animals were fasted overnight, but allowed free access to water.
Acute pancreatitis was established in 32 mice (AP group) by subcutaneous injection of cerulein (50 μg/kg dose) every hour for three hours. Sham group (32 mice) received subcutaneous injection of normal saline every hour for three hours. AP/PAF group (32 mice) received subcutaneous injection of cerulein (50 μg/kg dose) every hour for three hours, plus PAF (50 μg/kg dose) injected peritoneally immediately after the first cerulein challenge. AP/GAB group (32 mice) received subcutaneous injection of cerulein (50 μg/kg dose) every hour for three hours, plus therapeutic treatment with GAB (25 mg/kg i.p.) immediately after the first cerulein challenge.
The animals were sacrificed at 12 h after the first cerulein challenge or saline injection. Some mice (eight in every group) were decapitated and the blood was collected in vials for the analysis of serum amylase. Pancreata were dissected. Some were weighted and grounded for the analysis of MPO, and the others were fixed with formalin for histological scoring and immunohistochemical staining. Some left pancreatic tissues were dried and weighted.
MPO assay of pancreas was performed according to the instructions of commercial kit.
Serum amylase levels were measured using starch-iodine method.
Pancreas was dissected from its attachment and the fluid on its surface was dried with filter paper. Then the pancreas was placed in oven at a temperature of 90-92 °C for 10 h. Water content = (Weightwet-Weightdry)/Weightwet× 100%.
Pancreatic sections were stained with hematoxilin and eosin and graded in a blind manner by two investigators. Severity of pancreatitis was scored on a scale of 0 to 4 (normal to severity) in the categories (vacuolization, inflammation, edema) for a total score of 0 to 12, as described by J.Schmidt[4].
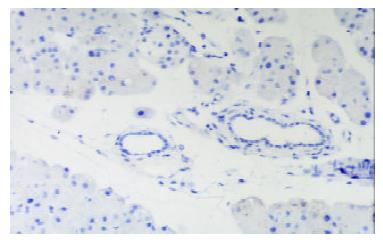
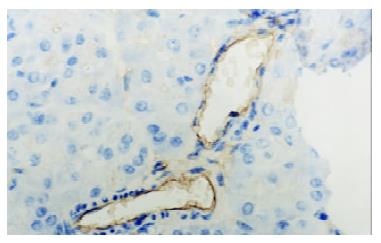
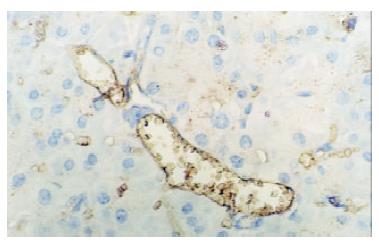
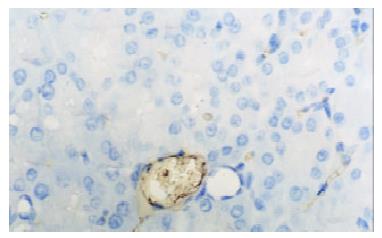
Pancreata were fixed in formalin for 12 h and then embedded in paraffin wax. Sections were cut at 4 μm in thickness and mounted on slides. They were deparaffinized by passing them through two changes of xylene and graded series of ethanol, followed by rinses in tap water and 0.01 mmol·L-1 phosphorate buffered saline (PBS),respectively. Endogenous peroxidase activity was quenched by treating the section with 3% hydrogen peroxide for 10 min. Nonspecific binding was blocked by incubating sections in 1% bovine albumin in PBS for 10 min, and then incubated for an hour in primary antibody (rabbit anti-E-selectin or rabbit anti-ICAM-1 polyclonal antibody). After rinsing in PBS, the sections were treated sequentially with biotinconjugated second antibody for 10 min and then with streptavidin-peroxidase for another 10 min with PBS rinsing after each step. Sections were stained subsequently with freshly prepared DAB reagent for 3 min, and terminated by rinsing in water. then the sections were immerged in hematoxylin for 3-5 min and 0.5 mmol·L-1 HCl for 10 s. Finally, when passed through two changes of xylene and graded series of alcohol, the sections were covered with coverslip for light analysis. Every slice was counted on ten different fields by two investigators independently. Vessel stained brown was considered positive vessel. (-), score 0, means no positive vessel was observed. (+), score 1, means 1-2 positive vessels were observed. (++), score 2, means 3-4 positive vessels were observed. (+++), score 3, means more than 5 positive vessels were observed.
Numerical variables are reported as means ± SEM and compared between groups using Newman-Keuls method. Rank data are reported as means and analyzed with Nemenyi method. Difference with P < 0.05 are considered significant.
In control group there was no staining of E-selectin and ICAM-1 in pancreatic venules. Expression of the two adhesion molecules was eminent in AP and AP/PAF. But there was no difference between the two groups. AP/GAB has a lower level of E-selectin and ICAM-1 expression compared with AP (Table 1, Figure 1, Figure 2, Figure 3, Figure 4).
Serum amylase levels and water content was used to evaluate the severity of pancreatic injury. Amylase rose at 12 h in AP and AP/PAF group against control group, but there was no difference between the two groups. GAB treatment significantly reduced the amylase level at 12 h (P < 0.05). Water content reflects the extent of pancreatic edema. At 12 h AP and AP/PAF showed a higher level of water content against sham and AP/GAB. AP/GAB still has a higher level of water content than sham (Table 2).
Measurement of neutrophil accumulation by MPO activity revealed that AP and AP/PAF had an increase of MPO activity almost 10-fold greater than sham. AP/GAB showed significantly lower level of MPO Activity than AP (P < 0.05). And this level did not return to the values seen in sham treated animals (Table 2).
Sham group was microscopically intact, with normal histological structure. Pancreatic lesions, such as edema, vacuation and infiltration of PMN were found in AP, AP/PAF and AP/GAB. There was a trend toward improvement in histological parameters in AP/GAB, but it failed to reach statistical significance. (Data not shown).
Platelet activating factor, 1-O-octadecyl-2-acetyl-Sn-glycero-3-phosphocholine, is a potent inflammatory mediator produced by endothelial cells, platelets, monocytes, neutrophils, and basophils. It has been widely studied that PAF played an important role in the pathogenesis of AP, and its local and systemic effects on AP have also been revealed. The systemic effects cause circulatory disturbances and multiple organ failure. Local effects include platelet aggregation, neutrophil accumulation, microvascular ischemia and increasing of capillary permeability[5-7]. Moreover, PAF itself also can induce pancreatitis when injected in peritoneal or superior pancreatoduodenal artery[8-10].
Accordingly, treatment of experimental pancreatitis with PAF antagonist has consistently shown significant local and systemic protection to reduce inflammatory changes[11-15]. But the mechanism of its protection has not been fully classified. One kind of PAF antagonist, lexipafant, has been studied in clinical trials in acute pancreatitis. In phase II and phase III clinical studies lexipafant showed better protection against AP, although a phase III clinical trial in UK showed little protection in the treatment of severe acute pancreatitis[16-18].
In vitro PAF can induce the expression of adhesion molecules[19], there was little information whether PAF and its antagonist could regulate the expression of adhesion molecules in AP. So, we conducted this experiment to testify the hypothesis.
Leukocyte accumulation from circulating blood to the site of inflammation includes multiple steps involving different kind of adhesion molecules on the endothelial cell. There are four steps in the adhesion cascade: (1) tethering; (2) triggering; (3) firm adhesion; (4) diapedesis- and these steps must occur in ordered sequence. Tethering interactions are the first step in the adhesion cascade. The molecules involved in this reaction come from the selectin family, such as E-selectin and P-selectin. The firm-binding phase involves superimmunoglobulin family, such as ICAM-1 and VCAM-1. Both E-selectin and ICAM-1 have shown to be necessary for transendothelial migration of polymorphonuclear leukocytes[20]. In AP, neutrophils and adhesion molecules elevate and play an important role[21,22].Treatment of severe acute pancreatitis with ICAM-1 monoclonal antibody reduces the severity of pancreatic and lung injury. The severity of AP is partially decreased in mice deficient in ICAM-1[22].
In our study adhesion molecules were elevated in AP. But additional PAF challenge couldn't increase the expression of adhesion molecules. PAF antagonist (GAB) can reduce the expression of adhesion molecules and reduce the accumulation of neutrophils in pancreas in AP. The mechanism may be that internal PAF has enough capability to induce the expression of adhesion molecule, and exogenous PAF do not have accelerating ability to induce more expression of adhesion molecules. PAF antagonist may have different ways in AP to reduce the expression of adhesion molecules: (1) reduce the concentration of cytokines, such as TNF-α and IL-1β[23,24]; (2) reduce oxidative stress in pancreas[24,25]; (3) repress the activation of NF-κB directly.
In conclusion, PAF plays an important role in the expression of adhesion molecules and accumulation of neutrophils in AP. PAF antagonist can be used early in mild AP. Further studies are necessary to determine whether PAF antagonist can prevent mild AP to transform into severe AP.
We gratefully acknowledge associate Prof. Di-Qing Zhang for his precious PAF antagonist (GAB).
Edited by Zhou YP
| 1. | Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504-512. [PubMed] |
| 2. | Blackstone MO. ICAM-1 in cerulein-induced pancreatitis. Gastroenterology. 1999;117:748-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Tani S, Otsuki M, Itoh H, Fujii M, Nakamura T, Oka T, Baba S. Histologic and biochemical alterations in experimental acute pancreatitis induced by supramaximal caerulein stimulation. Int J Pancreatol. 1987;2:337-348. [PubMed] |
| 4. | Schmidt J, Lewandrowski K, Fernandez-del Castillo C, Mandavilli U, Compton CC, Warshaw AL, Rattner DW. Histopathologic correlates of serum amylase activity in acute experimental pancreatitis. Dig Dis Sci. 1992;37:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Johnson CD. Platelet-activating factor and platelet-activating factor antagonists in acute pancreatitis. Dig Surg. 1999;16:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Zhao LY, Chen CY, Liang P, Chen LY. ICAM-1 and VCAM-1 expressions on benign gastric mucosa and gastric adenocarcinoma associated with Helicobacter pylori infection. Shijie Huaren Xiaohua Zazhi. 2000;8:279-281. |
| 7. | Zhao XY, Xia SH. Platelet-activating factor and onset and treatment of acute pancreatitis. Shijie Huaren Xiaohua Zazhi. 2001;9:958-960. |
| 8. | Konturek SJ, Dembinski A, Konturek PJ, Warzecha Z, Jaworek J, Gustaw P, Tomaszewska R, Stachura J. Role of platelet activating factor in pathogenesis of acute pancreatitis in rats. Gut. 1992;33:1268-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Kald B, Kald A, Ihse I, Tagesson C. Release of platelet-activating factor in acute experimental pancreatitis. Pancreas. 1993;8:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Emanuelli G, Montrucchio G, Gaia E, Dughera L, Corvetti G, Gubetta L. Experimental acute pancreatitis induced by platelet activating factor in rabbits. Am J Pathol. 1989;134:315-326. [PubMed] |
| 11. | Werner J, Z'graggen K, Fernández-del Castillo C, Lewandrowski KB, Compton CC, Warshaw AL. Specific therapy for local and systemic complications of acute pancreatitis with monoclonal antibodies against ICAM-1. Ann Surg. 1999;229:834-840; discussion 841-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Rau B, Paszkowski A, Esber S, Gansauge F, Poch B, Beger HG, Möller P. Anti-ICAM-1 antibody modulates late onset of acinar cell apoptosis and early necrosis in taurocholate-induced experimental acute pancreatitis. Pancreas. 2001;23:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Rau B, Bauer A, Wang A, Gansauge F, Weidenbach H, Nevalainen T, Poch B, Beger HG, Nussler AK. Modulation of endogenous nitric oxide synthase in experimental acute pancreatitis: role of anti-ICAM-1 and oxygen free radical scavengers. Ann Surg. 2001;233:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Eibl G, Buhr HJ, Foitzik T. Therapy of microcirculatory disorders in severe acute pancreatitis: what mediators should we block. Intensive Care Med. 2002;28:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Lundberg AH, Fukatsu K, Gaber L, Callicutt S, Kotb M, Wilcox H, Kudsk K, Gaber AO. Blocking pulmonary ICAM-1 expression ameliorates lung injury in established diet-induced pancreatitis. Ann Surg. 2001;233:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kingsnorth AN, Galloway SW, Formela LJ. Randomized, double-blind phase II trial of Lexipafant, a platelet-activating factor antagonist, in human acute pancreatitis. Br J Surg. 1995;82:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | McKay CJ, Curran F, Sharples C, Baxter JN, Imrie CW. Prospective placebo-controlled randomized trial of lexipafant in predicted severe acute pancreatitis. Br J Surg. 1997;84:1239-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, Toh SK, Skaife P, Leeder PC, Wilson P. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Chihara J, Maruyama I, Yasuba H, Yasukawa A, Yamamoto T, Kurachi D, Mouri T, Seguchi M, Nakajima S. Possible induction of intercellular adhesion molecule-1 (ICAM-1) expression on endothelial cells by platelet-activating factor (PAF). J Lipid Mediat. 1992;5:159-162. [PubMed] |
| 20. | Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2222] [Cited by in RCA: 2146] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 21. | Zaninovic V, Gukovskaya AS, Gukovsky I, Mouria M, Pandol SJ. Cerulein upregulates ICAM-1 in pancreatic acinar cells, which mediates neutrophil adhesion to these cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G666-G676. [PubMed] |
| 22. | Frossard JL, Saluja A, Bhagat L, Lee HS, Bhatia M, Hofbauer B, Steer ML. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 200] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Luscinskas FW, Cybulsky MI, Kiely JM, Peckins CS, Davis VM, Gimbrone MA. Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991;146:1617-1625. [PubMed] |
| 24. | Lane JS, Todd KE, Gloor B, Chandler CF, Kau AW, Ashley SW, Reber HA, McFadden DW. Platelet activating factor antagonism reduces the systemic inflammatory response in a murine model of acute pancreatitis. J Surg Res. 2001;99:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Wang WX, Zhao HP, Shou NY, Yang CW. Role of oxygen free radical and other inflammatory mediators in acute necrotic pancreatitis. World J Gastroenterol. 1998;4:59. |