Published online Feb 15, 2003. doi: 10.3748/wjg.v9.i2.271
Revised: January 4, 2002
Accepted: March 18, 2002
Published online: February 15, 2003
AIM: Many growth factors, such as epidermal growth factor (EGF), are associated with the carcinogenesis. EGF plays its role in the proliferation of hepatoma cells through binding with EGF receptor (EGFR) and a series of signal transduction. But the postreceptor pathway is still not clear. In the present experiment, we studied the effect of tyrosine kinase, protein kinase C, Na+/H+ exchange, calmodulin and voltage-dependent Ca2+ channel on EGF-induced hepatoma cell proliferation.
METHODS: Hepatoma cell line SMMC7721 was cultured in RPMI1640 serum-free medium. In order to study the effect of thyrosine kinase, protein kinase C, Na+/H+ exchange, calmodulin and voltage-dependent Ca2+ channel on human heptoma cell proliferation induced by epidermal growth factor (EGF), DNA synthesis rate of hepatoma cells was measured by the method of 3H-TdR incorporation.
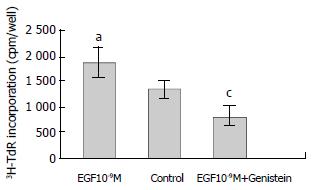
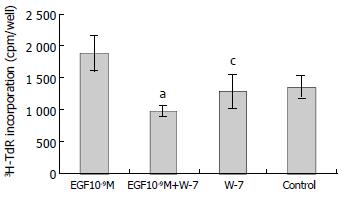
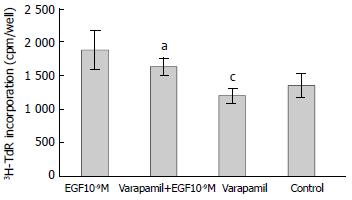
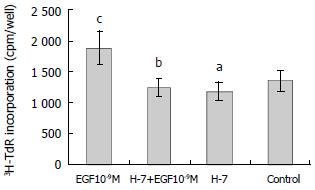
RESULTS: EGF (10-9 M) stimulated the proliferation of heptoma cells significantly (3H-TdR incorporation was 1880 ± 281 cpm/well, P < 0.05), and this effect was significantly inhibited by tyrosine kinase inhibitor genistein (3H-TdR incorporation was 808 ± 209 cpm/well, P < 0.001). Calmodulin inhibitor W-7, protein kinase C inhibitor H-7 and Na+/H+ exchange inhibitor amiloride individually had significant inhibiting effect on EGF-induced proliferation of hepatoma cells (3H-TdR incorporation was 978 ± 87.3 cpm/well, 1241 ± 147 cpm/well, 1380 ± 189 cpm/well, respectivly, P < 0.001, P < 0.01, P < 0.05), but they all had no effect on the basal level proliferation of cultured hepatoma cells (3H-TdR incorporation was 1284 ± 260 cpm/well, 1179 ± 150 cpm/well, 1392 ± 152 cpm/well, respectivly, 3H-TdR incorporation of the control was 1353 ± 175 cpm/well, P > 0.05). Voltage-dependent Ca2+ channel inhibitor verapamil had no inhibition on EGF-induced proliferation of hepatoma cells (3H-TdR incorporation was 1637 ± 133 cpm/well, P > 0.05), it also had no effect on the basal level proliferation of cultured hepatoma cells (3H-TdR incorporation was 1196 ± 12 cpm/well, P > 0.05).
CONCLUSION: Our data suggest that tyrosine kinase, Ca2+-calmodulin-dependent pathway, protein kinase C and Na+/H+ exchange play a critical role in EGF-induced proliferation of hepatoma cells and that the effect of EGF is independent of voltage-dependent Ca2+ channel.
- Citation: Wu BW, Wu Y, Wang JL, Lin JS, Yuan SY, Li A, Cui WR. Study on the mechanism of epidermal growth factor-induced proliferation of hepatoma cells. World J Gastroenterol 2003; 9(2): 271-275
- URL: https://www.wjgnet.com/1007-9327/full/v9/i2/271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i2.271
Most hepatocellular carcinoma (HCC) is associated with cirrhosis, a condition in which liver cell necrosis coexists with inflammation and hepatocellular regeneration. HCC arising in cirrhotic livers is exposed to a number of growth factors mediating hepatocellular regeneration. Epidermal growth factor (EGF) is one of them.
EGF is a single strand polypeptide composed of 53 amino acid and has implicated in the regulation of a wide variety of physiological and pathological processes including embryo genesis, growth, tissue repair, regeneration and neoplasia. EGF inhibits gastric acid secretion and has a cytoprotective effect on stomach tissue, EGF has been of clinical interest for the treatment of acid hypersecretion and the healing of ulcers. EGF has been implicated as a hepatotrophic factor during liver regeneration, has protective effect during hepatic damage through inducing the DNA synthesis of hepatic cells[1-4]. EGF also induces DNA synthesis of hepatoma cells and promotes the occurrence and expansion of hepatoma[5,6].
EGF exerts its biologic effects by binding with the EGF receptor (EGFR), a type of transmembrane glucoprotein with a molecule of 170kD. The primary structure of its intramembranous part is associated with the expression of oncogene erb-B. The intracellular part of EGFR has tyrosine kinase activity. EGFR is overexpressed in various carcinoma, such as hepatoma[7-9], gastric carcinoma[10-14], colon carcinoma[15-20], lung carcinoma[21-23] and prostatic carcinoma[24-28], and also associated with the histologic types and invasiveness of the tumor[29-32]. Many growth factors have the effect of promoting cells proliferation and malignant transformation by binding with EGFR such as EGF, heparin binding EGF (HB-EGF)[33,34], transforming growth factor β (TGF-β)[35,36] and hepatopoientin[37,38]. After the integration of HBV-X gene into hepatic cells, EGFR was activated and overexpressed, which induced liver carcinogenesis[39].
In general, EGF induces tumor cell division and proliferation through a series of signal transduction by binding with EGF receptor (EGFR) and activating its thyrosine protein kinase and phosphorating itself and its protein substrate[40]. This postreceptor singnal transduction is associated with phosphatidylinositol pathway[41]. But the exact mechanism is still not clear.
In this report, we have characterized the anti-tumor activity of tyrosine kinase, calmodulin, protein kinase C, Na+/H+ exchange and voltage-dependent Ca2+ channel inhibitors. The SMMC7721 cell line cultured in serum-free medium was used in this study.
All chemicals were purchased from sigma chemical CO. (St.Louis. Mo. USA) unless otherwise stated. 3H-TdR was purchased from the Institute of Nuclear Power of China. Verapamil, streptomycin, penicillin and 0.5% hydrocortisone were clinical medicines.
SMMC7721 cells were stored in our laboratory and cultured in RPMI1640 serum-free medium. Briefly, SMMC7721 cells were grown in RPMI1640 supplemented with 5 μg/mL transferrin, 20 μU/mL insulin, 0.4 μg/mL hydrocortisone and 100 μg/mL streptomycin and penicillin.
Eleven groups were separated in the experiment: (1) control, (2) EGF (10-9M), (3) W-7, (4) verapamil, (5) W-7 + EGF, (6) verapamil + EGF, (7) genistein + EGF, (8) H-7, (9) H-7 + EGF, (10) amiloride, (11) amiloride + EGF.
The DNA synthesis rates were measured by the method of 3H-TdR incorporation. The hepatoma cells were seeded into 96 well plates at a density of 1 × 104/well and incubated with serum-free RPMI1640. After 24 h, fresh medium was changed, and reagents added. After incubation for 18 h the medium was then replaced with fresh medium containing 3H-TdR 0.5 μci/mL for another 6 h. The cells were finally lysed with 0.33 mol/L HCl, 3H-TdR incorporation was determined with a β-counter.
Two-side t test was used to examine the significant difference between groups.
Genistein is a tyrosine kinase inhibitor. EGF (10-9M) stimulated the growth of hepatoma cells significantly compared with the control (P < 0.05). EGF-induced 3H-TdR incorporation was reduced from 1880 ± 281 cpm/well to 808 ± 109 cpm/well by genistein (P < 0.001, Figure 1).
W-7 is a calmodulin inhibitor. W-7 (25 μmol/L) alone had no effect on 3H-TdR incorporation, the incorporation of W-7 group and control were 1284 ± 260 cpm/well and 1353 ± 175 cpm/well, respectively (P > 0.05). When W-7 was added with EGF (10-9M), the effect of EGF on the growth of hepatoma cells was inhibited significantly, the incorporation (978 ± 87.3 cpm/well) was significantly lower than that of EGF (10-9M) group (1880 ± 281 cpm/well) (P < 0.001, Figure 2).
Verapamil is voltage-dependent Ca2+ channel inhibitor. As shown in Figure 3, verapamil (100 μmol/L) alone had no effect on 3H-TdR incorporation, the incorporation of verapamil group and control were 1196 ± 112 cpm/well and 1353 ± 175 cpm/well, respectively (P > 0.05). When verapamil (100 μmol/L) was used with EGF (10-9M), the incorporation was 1637 ± 133 cpm/well, which was not significantly different from the incorporation with EGF (10-9M) alone (1880 ± 281 cpm/well, P > 0.05).
H-7 is a specific inhibitor of protein kinase C. H-7 (50 μmol/L) alone had no effect on the basal level 3H-TdR incorporation, the incorporation of H-7 group and control were 1179 ± 150 cpm/well and 1353 ± 175 cpm/well, respectively (P > 0.05). When H-7 was used together with EGF (10-9M), the effect of EGF on the proliferation of hepatoma cells was inhibited significantly, the incorporation (1241 ± 147 cpm/well) was significantly lower than that of EGF group (1880 ± 281 cpm/well, P < 0.01) (Figure 4).
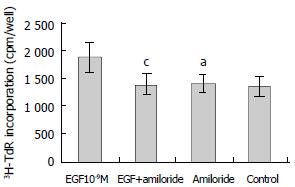
Amiloride is an inhibitor of Na+/H+ exchange. As shown in Figure 5, amiloride (0.1 mmol/L) alone had no effect on 3H-TdR incorporation, the incorporation of amiloride group and control were 1392 ± 152 cpm/well and 1353 ± 175 cpm/well, respectively (P > 0.05). When amiloride (0.1 mmol/L) was used with EGF (10-9M), the effect of EGF on the proliferation of hepatoma cells was inhibited significantly, the incorporation (1380 ± 189 cpm/well) was significantly lower than that of EGF group (1880 ± 281 cpm/well, P < 0.05) (Figure 5).
In this study, we demonstrated that EGF (10-9M) had strong stimulation on the growth of hepatoma cells. It is well documented that EGF binds with the EGFR and activates the tyrosine kinase of the receptor. In this paper, the tyrosine kinase inhibitor genistein effectively blocked the growth of hepatoma cells induced by EGF.
Introcellular Ca2+ is one of critical factors maintaining the intracellular homeostasis. The concentration of Ca2+ in the cell plasma is about 10-7 mol/L, which is far lower than extracellular concentration of 10-3 mol/L. The enhancement of the intracellular Ca2+ concentration is achieved in two ways: one is the membrane depolarization, the other is the release from the intracellular stores intervened by phosphatidylinositol 1,4,5-triphosphate (IP3). When the concentration of the intracellular Ca2+ rises to 5 folds above normal, the Ca2+ pump will be activated and put the Ca2+ out of the cell. Intracellular Ca2+ is the cofactor of many critical enzymes and functional proteins, and is an important intracellular messenger.
Studies demonstrate that in hepatoma cells EGF and other Ca2+ mobilizing hormones stimulate the rapid formation of IP3 and a concomitant rise in cytosolic Ca2+, apparently mobilized from intracellular stores[42]. Ca2+-binding protein regucalcin plays a role in the maintenance of intracellular Ca2+ homeostasis, and inhibits Ca2+-activated DNA fragmentation. Regucalcin may play a physiological role in the control of over-prolifertive cells[43].
In this experiment, the calmodulin inhibitor W-7 was used with EGF, and it significantly inhibited the growth of hepatoma cells induced by EGF, but it showed no effect on the growth of hepatoma cell when used alone. On the contrary, voltage-dependent Ca2+ channel inhibitor verapamil had no effect on the growth of hepatoma cells induced by EGF. It is suggested that Ca2+-calmodulin-dependent pathway plays a critical role on the proliferation of heptoma cells induced by EGF, and the effect of EGF is independent of voltage-dependent Ca2+ channel.
In the signal transduction pathway of phosphatidylinositol, activators bind with the cell surface receptor and activate phospholipase C (PLC)-β through G protein. PLC catalyzes dehydration of phosphatidylinositol 4,5-bisphosphate (PIP2) into IP3 and diglyceride (DG). IP3 promotes the release of Ca2+ from endoplasmic reticulum. With the participation of Ca2+, DG activates PKC, and PKC urges the phosphoration of tyrosine which activates oncogene erb-B with productions of protein Erb-B2 and Erb-B3[44]. In this study, PKC inhibitor H-7 used alone had no significant effect compared with control, but when used with EGF, it significantly inhibited the mitogenic effect of EGF on hepatoma cells. The result suggests the activation of PKC is an important link in the effect of EGF, and also implies that the effect of EGF is associated with phosphatidylinositol signaling pathway.
Intracellular pH (pHi) is another critical factor maintaining the intracellular homeostasis. At present, many studies found that pHi is associated with the pathogenesis of many diseases and mitosis of many kind of cells. Na+/H+ exchange is an important mechanism of pHi modulation. Activation of Na+/H+ exchange is the trigger of cellular protein and DNA synthesis, and is of fundamental importance for tumor growth. An increased influx of Na+ through the stimulation of Na+/H+ exchange plays a key role during early phases of the cell cycle. Na+/H+ exchange messenger RNA expression may reach 10 times higher in HepG2 than in normal hepatocytes. EGF can increase baseline pHi in a dose-dependent manner. This effect was completely inhibited by pretreatment with amiloride[45]. In this experiment, we found that Na+/H+ exchange inhibitor amiloride (0.1 mmol/L) alone showed no effect on the basal level 3H-TdR incorporation of hepatoma cells. On the contrary, when it was used with EGF (10-9M), the stimulatory effect of EGF was significantly inhibited. This suggests that the activation of Na+/H+ exchange may be one pathway of the effect of EGF.
Recent studies have shown that Ca2+/calmodulin-dependent protein kinase IV was overexpressed in HCC with high activity and might be involved in the development of HCC[46]. The effect of EGF on the hepatoma cells was associated with protein kinase C and calmodulin. Protein kinase C can activate Na+/H+ exchange. After the phosphorylation of EGFR, the proliferation signal was sent into the nucleus through STAT3 (signal transducers and activators of transcription 3)[47]. The mechanism of this signal transduction is very complicated. It is connected with the activation of oncogene ras[48,49], erb-B[44], cyclinD1 and c-Myc[50,51]. The effect of cell proliferation induced by EGF is associated with the MAPK (mitogen-activated protein kinase) pathway. The interactions between them demand more intensive studies.
In conclusion, our data suggest that PKC, calmodulin and Na+/H+ exchange could play a critical role in the growth of some hepatic tumors and that the inhibition of PKC, calmodulin or Na+/H+ exchange activity through pharmacological or genetic interventions, could theoretically be a useful strategy to reduce the growth rate of hepatocellular carcinoma.
Edited by Pang LH
| 1. | Diehl AM, Rai RM. Liver regeneration 3: Regulation of signal transduction during liver regeneration. FASEB J. 1996;10:215-227. [PubMed] |
| 2. | Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995;9:1527-1536. [PubMed] |
| 3. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2467] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 4. | Yan JP, Jia JB, Ma XH, Wu XR, Zhao YC, Han DW. Immunohistochemical study on expression of epidermal growth factor receptor at hepatocyte nuclei in experimental rat liver cirrhosis. Huaren Xiaohua Zazhi. 1998;6:15-16. |
| 5. | Kong YG, Zhen J, Lu GJ, Xu SB, Chen YF. The effects of EGF on the growth of human hepatoma cells and the regulatory action of somatostatin on EGF receptors. Zhonghua Xiaohua Zazhi. 1995;15:129-132. |
| 6. | Hisaka T, Yano H, Haramaki M, Utsunomiya I, Kojiro M. Expressions of epidermal growth factor family and its receptor in hepatocellular carcinoma cell lines: relationship to cell proliferation. Int J Oncol. 1999;14:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Moser GJ, Wolf DC, Goldsworthy TL. Quantitative relationship between transforming growth factor-alpha and hepatic focal phenotype and progression in female mouse liver. Toxicol Pathol. 1997;25:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Kira S, Nakanishi T, Suemori S, Kitamoto M, Watanabe Y, Kajiyama G. Expression of transforming growth factor alpha and epidermal growth factor receptor in human hepatocellular carcinoma. Liver. 1997;17:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | DeCicco LA, Kong J, Ringer DP. Carcinogen-induced alteration in liver epidermal growth factor receptor distribution during the promotion stage of hepatocarcinogenesis in rat. Cancer Lett. 1997;111:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Peláez Buján Mdel C, Ruibal Morell A, Aza González J. Gastric carcinoma: Expression of c-erbB-2/neu oncoprotein, epidermal growth factor receptor, cathepsin D, progesterone receptor and tumor associated glycoprotein-72 in different histological types. Rev Esp Enferm Dig. 1999;91:826-837. [PubMed] |
| 11. | Koyama S, Maruyama T, Adachi S. Expression of epidermal growth factor receptor and CD44 splicing variants sharing exons 6 and 9 on gastric and esophageal carcinomas: A two-color flow-cytometric analysis. J Cancer Res Clin Oncol. 1999;125:47-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Pai R, Wyle FA, Cover TL, Itani RM, Domek MJ, Tarnawski AS. Helicobacter pylori culture supernatant interferes with epidermal growth factor-activated signal transduction in human gastric KATO III cells. Am J Pathol. 1998;152:1617-1624. [PubMed] |
| 13. | Wang HT, Chen BW, Jia BQ. Roles of epidermal growth factor (EGF) and EGF receptor in gastric cancer. Xin Xiaohuabingxue Zazhi. 1997;5:393-394. |
| 14. | Sanz-Ortega J, Steinberg SM, Moro E, Saez M, Lopez JA, Sierra E, Sanz-Esponera J, Merino MJ. Comparative study of tumor angiogenesis and immunohistochemistry for p53, c-ErbB2, c-myc and EGFr as prognostic factors in gastric cancer. Histol Histopathol. 2000;15:455-462. [PubMed] |
| 15. | Baba I, Shirasawa S, Iwamoto R, Okumura K, Tsunoda T, Nishioka M, Fukuyama K, Yamamoto K, Mekada E, Sasazuki T. Involvement of deregulated epiregulin expression in tumorigenesis in vivo through activated Ki-Ras signaling pathway in human colon cancer cells. Cancer Res. 2000;60:6886-6889. [PubMed] |
| 16. | Liu B, Fang M, Schmidt M, Lu Y, Mendelsohn J, Fan Z. Induction of apoptosis and activation of the caspase cascade by anti-EGF receptor monoclonal antibodies in DiFi human colon cancer cells do not involve the c-jun N-terminal kinase activity. Br J Cancer. 2000;82:1991-1999. [PubMed] |
| 17. | Hao YR, Ding GY, Chen PY, Geng JH. Effect of EGF receptor expression on cell proliferation in colorectal cancer. Huaren Xiaohua Zazhi. 1998;6:313-314. |
| 18. | Wang Q, Wu JS, Gao DM, Lai DN, Ma QJ. Expression significance of epidermal growth factor receptor and transforming growth factor a mRNA in human colorectal carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:590-592. |
| 19. | Porebska I, Harlozińska A, Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol. 2000;21:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Di Carlo A, Mariano A, D'Alessandro V, Belli G, Romano G, Macchia V. Evaluation of epidermal growth factor receptor, carcinoembryonic antigen and Lewis carbohydrate antigens in human colorectal and liver neoplasias. Oncol Rep. 2001;8:387-392. [PubMed] |
| 21. | Reissmann PT, Koga H, Figlin RA, Holmes EC, Slamon DJ. Amplification and overexpression of the cyclin D1 and epidermal growth factor receptor genes in non-small-cell lung cancer. Lung Cancer Study Group. J Cancer Res Clin Oncol. 1999;125:61-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Schneider J, Presek P, Braun A, Bauer P, Konietzko N, Wiesner B, Woitowitz HJ. p53 protein, EGF receptor, and anti-p53 antibodies in serum from patients with occupationally derived lung cancer. Br J Cancer. 1999;80:1987-1994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | López-Guerrero JA, Bolufer-Gilabert P, Vera-Sempere FJ, Marugán de la Concha I, Barragán-González E. C-erbB-2 expression and its relationship with ploidy, p53 abnormalities and epidermal growth factor receptor content in human non-small cell lung cancer. Clin Chim Acta. 1999;285:105-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Kumar VL, Majumder PK, Kumar V. Observations on EGFR gene amplification and polymorphism in prostatic diseases. Int Urol Nephrol. 2000;32:73-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | De Miguel P, Royuela R, Ruiz A, Fraile B, Paniagua R. Immunohistochemical comparative analysis of transforming growth factor alpha, epidermal growth factor, and epidermal growth factor receptor in normal, hyperplastic and neoplastic human prostates. Cytokine. 1999;11:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Richter F, Huang HF, Li MT, Danielpour D, Wang SL, Irwin RJ. Retinoid and androgen regulation of cell growth, epidermal growth factor and retinoic acid receptors in normal and carcinoma rat prostate cells. Mol Cell Endocrinol. 1999;153:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Sundareshan P, Nagle RB, Bowden GT. EGF induces the expression of matrilysin in the human prostate adenocarcinoma cell line, LNCaP. Prostate. 1999;40:159-166. [PubMed] [DOI] [Full Text] |
| 28. | Gil-Diez de Medina S, Salomon L, Colombel M, Abbou CC, Bellot J, Thiery JP, Radvanyi F, Van der Kwast TH, Chopin DK. Modulation of cytokeratin subtype, EGF receptor, and androgen receptor expression during progression of prostate cancer. Hum Pathol. 1998;29:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Takita M, Onda M, Tokunaga A. Immunohistochemical demonstration of angiogenic growth factors and EGF receptor in hepatic metastases and primary human gastric cancer. Nihon Ika Daigaku Zasshi. 1998;65:358-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Liu F, Qi HL, Chen HL. Effects of all-trans retinoic acid and epidermal growth factor on the expression of nm23-H1 in human hepatocarcinoma cells. J Cancer Res Clin Oncol. 2000;126:85-90. [PubMed] |
| 31. | Parker C, Roseman BJ, Bucana CD, Tsan R, Radinsky R. Preferential activation of the epidermal growth factor receptor in human colon carcinoma liver metastases in nude mice. J Histochem Cytochem. 1998;46:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | De Jong KP, Stellema R, Karrenbeld A, Koudstaal J, Gouw AS, Sluiter WJ, Peeters PM, Slooff MJ, De Vries EG. Clinical relevance of transforming growth factor alpha, epidermal growth factor receptor, p53, and Ki67 in colorectal liver metastases and corresponding primary tumors. Hepatology. 1998;28:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Kiso S, Kawata S, Tamura S, Miyagawa J, Ito N, Tsushima H, Yamada A, Umeki S, Higashiyama S, Taniguchi N. Expression of heparin-binding epidermal growth factor-like growth factor in the hepatocytes of fibrotic rat liver during hepatocarcinogenesis. J Gastroenterol Hepatol. 1999;14:1203-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Inui Y, Higashiyama S, Kawata S, Tamura S, Miyagawa J, Taniguchi N, Matsuzawa Y. Expression of heparin-binding epidermal growth factor in human hepatocellular carcinoma. Gastroenterology. 1994;107:1799-1804. [PubMed] |
| 35. | Zhang J, Wang WL, Li Q, Qiao Q. Expression and significance of transforming growth factor-α and its receptor in human primary hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:939-942. |
| 36. | Harada K, Shiota G, Kawasaki H. Transforming growth factor-alpha and epidermal growth factor receptor in chronic liver disease and hepatocellular carcinoma. Liver. 1999;19:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Wang G, Yang X, Zhang Y, Wang Q, Chen H, Wei H, Xing G, Xie L, Hu Z, Zhang C. Identification and characterization of receptor for mammalian hepatopoietin that is homologous to yeast ERV1. J Biol Chem. 1999;274:11469-11472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Li Y, Li M, Xing G, Hu Z, Wang Q, Dong C, Wei H, Fan G, Chen J, Yang X. Stimulation of the mitogen-activated protein kinase cascade and tyrosine phosphorylation of the epidermal growth factor receptor by hepatopoietin. J Biol Chem. 2000;275:37443-37447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Miyaki M, Sato C, Sakai K, Konishi M, Tanaka K, Muraoka M, Kikuchi-Yanoshita R, Nadaoka Y, Kanda H, Kitagawa T. Malignant transformation and EGFR activation of immortalized mouse liver epithelial cells caused by HBV enhancer-X from a human hepatocellular carcinoma. Int J Cancer. 2000;85:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Francavilla A, Barone M, Azzarone A, Panella C, Giangaspero A. Cell regeneration in the pathobiology of liver carcinomas. Ital J Gastroenterol. 1991;23:589-593. [PubMed] |
| 41. | Hung WC, Chuang LY, Tsai JH, Chang CC. Effects of epidermal growth factor on growth control and signal transduction pathways in different human hepatoma cell lines. Biochem Mol Biol Int. 1993;30:319-328. [PubMed] |
| 42. | Gilligan A, Prentki M, Glennon C, Knowles BB. Epidermal growth factor-induced increases in inositol trisphosphates, inositol tetrakisphosphates, and cytosolic Ca2+ in a human hepatocellular carcinoma-derived cell line. FEBS Lett. 1988;233:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Nakajima M, Murata T, Yamaguchi M. Expression of calcium-binding protein regucalcin mRNA in the cloned rat hepatoma cells (H4-II-E) is stimulated through Ca2+ signaling factors: involvement of protein kinase C. Mol Cell Biochem. 1999;198:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Emkey R, Kahn CR. Cross-talk between phorbol ester-mediated signaling and tyrosine kinase proto-oncogenes. II. Comparison of phorbol ester and sphingomyelinase-induced phosphorylation of ErbB2 and ErbB3. J Biol Chem. 1997;272:31182-31189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Strazzabosco M, Poci C, Spirlì C, Zsembery A, Granato A, Massimino ML, Crepaldi G. Intracellular pHregulation in Hep G2 cells: Effects of epidermal growth factor, transforming growth factor-alpha, and insulinlike growth factor-II on Na+/H+ exchange activity. Hepatology. 1995;22:588-597. [PubMed] |
| 46. | Tamura N, Tai Y, Sugimoto K, Kobayashi R, Konishi R, Nishioka M, Masaki T, Nagahata S, Tokuda M. Enhanced expression and activation of Ca (2+)/calmodulin-dependent protein kinase IV in hepatocellular carcinoma. Cancer. 2000;89:1910-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 47. | Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, Tweardy DJ. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 402] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 48. | Zheng XL, Matsubara S, Diao C, Hollenberg MD, Wong NC. Epidermal growth factor induction of apolipoprotein A-I is mediated by the Ras-MAP kinase cascade and Sp1. J Biol Chem. 2001;276:13822-13829. [PubMed] |
| 49. | Wang Q, Lin ZY, Feng XL. Alterations in metastatic properties of hepatocellular carcinoma cell following H-ras oncogene transfection. World J Gastroenterol. 2001;7:335-339. [PubMed] |
| 50. | Ramljak D, Jones AB, Diwan BA, Perantoni AO, Hochadel JF, Anderson LM. Epidermal growth factor and transforming growth factor-alpha-associated overexpression of cyclin D1, Cdk4, and c-Myc during hepatocarcinogenesis in Helicobacter hepaticus-infected A/JCr mice. Cancer Res. 1998;58:3590-3597. [PubMed] |
| 51. | Niu ZS, Li BK, Wang M. Expression of p53 and C-myc genes and its clinical relevance in the hepatocellular carcinomatous and pericarcinomatous tissues. World J Gastroenterol. 2002;8:822-826. [PubMed] |