INTRODUCTION
Angiogenesis, the process of new blood vessel formation from existing vessels, is essential for tumor progression and metastasis[1-7]. Tumor cells stimulate angiogenesis by secretion of angiogenic factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)[8]. At the same time, tumor cells also produce anti-angiogenic factors, including angiostatin[9] and endostatin[10]. Tumor angiogenesis is dependent upon the local balance of these positive and negative regulators[11]. Inhibition of angiogenesis has been shown to inhibit local tumor growth and metastasis[12]. Systemic administration of endostatin in tumor-bearing mice has been shown to keep the primary tumors in a dormant state[10], thereby preventing tumor metastasis.
Clinical trials involving angiostatin and endostatin require large quantities of recombinant proteins, which are difficult to produce. In addition, these proteins do not maintain their bioactivity and are hard to be administered systemically. Gene therapy with endostatin employing a viral delivery system has shown a high efficacy in inhibiting tumor growth and metastasis in mice[13-15]. However, the safety issues in its usage make this approach less attractive in human clinical trials. Nonviral gene therapy with endostatin was reported[16,17], but the efficiency was rather low as compared with viral delivery systems. The use of a cationic liposome-mediated gene transfer system together with a transferrin ligand achieved high transfection efficiency upon delivery of the p53 gene in mice[18]. In this study, we evaluated the effect of aerosol administration of TF-liposome-endostatin on liver tumor growth in mice. The results indicated that TF-liposome-mediated endostatin gene therapy strongly inhibited angiogenesis and the growth of mouse xenograft liver tumors.
MATERIALS AND METHODS
Plasmid construction
The endostatin gene was obtained by PCR from a human liver cDNA library (Clontech Co). Endostatin was digested with EcoRI/XbaI and cloned in the EcoRI/XbaI site of pcDNA3.0 (Invitrogen Co). The sequence encoding the signal peptide from alkaline phosphatase[19] was inserted into the HindIII/EcoRI site upstream of endostatin and an epitope tag derived from influenza virus hemagglutinin A (HA) was fused in the EcoRI site between the signal peptide and endostatin. Recombinant plasmid was purified by QIA prep spin miniprep kit (QIAGEN Co).
Preparation of liposomes
Dried lipid films were prepared by solubilizing stearylamine, phosphatidyl choline and cholesterol at a 2:7:1 molar ratio in chloroform and subsequent removal of the chloroform by evaporation[20]. The films were hydrated for 10 min with sterile water at 60 °C and then vortexed to generate liposomes. After hydration, the liposomes were sonicated on ice and filtered (pore size 0.22 mm). The diameter of liposome was assessed under scanning electron microscopy.
Formation of the TF-liposome-plasmid complex and determination of the encapsulation coefficency
The TF-liposome-plasmid complexes were prepared using 10:1:1, 10:5:1, 10:10:1 or 10:20:1 (mg:nmol:mg) ratios of TF:liposome:DNA. Liposomes and TF were mixed and incubated for 20 min at room temperature. The purified plasmid was then added to the solution, mixed immediately and thoroughly, and incubated for a minimum of 20 min at room temperature. The encapsulation coefficency of the TF-liposome-plasmid complexes was determined by 7.5 g·L-1 agarose electrophoresis. The complexes were dissociated with 4 g·L-1 SDS.
Expression of endostatin in vitro
HUVEC cells were obtained from Shanghai Cellular Research Institute and maintained in 1640 medium supplemented with 100 mL·L-1 fetal bovine serum. HUVEC cells were plated in six-well plates at 2.5 × 105 cells/well, and transfected with TF-liposome-plasmid complex containing 2 μg of purified plasmid. The conditioned media were collected after 48 h and concentrated in a microconcentrator (Amicon Co). And the cells were harvested by centrifugation, mixed with sample buffer and treated with sonic. The samples were subjected to SDS-PAGE using 120 g·L-1 polyacrylamide gels, followed by Western blot analysis. The primary monoclonal mouse-anti-HA antibody (Babco Co) was used at 1:1000 dilution followed by goat anti-mouse IgG-HRP (Sino-American Biotechnology Co) at 1:50 dilution. The membrane was stained by DAB.
Treatment of tumor-bearing mice with TF-liposome-pcDNA3.0/endostatin complexes
BALB/c male and female mice (obtained from the Animal Center of Nanjing Medical University, China) weighing about 20 grams were used in this study. Mice were fed with a standard rodent diet. Heps mouse liver tumor[21] (obtained from the Animal Center of Chinese Academy of Sciences in Shanghai, China) was minced thoroughly and cancer cells were obtained at a concentration of 5 × 106 cells/mL. A total of 1 × 106 tumor cells were inoculated under the skin of the right thigh for each mouse. After 72 h inoculation, the tumor-bearing mice were treated with the aerosol containing 50, 250 or 500 μg DNA/kg of TF-liposome-pcDNA3.0/endostatin complexes (in 100 μL) or 500 μg DNA/kg of TF-liposome-pcDNA3.0 vector complexes every 72 h, for a total of five administrations. After 72 h of the fifth administration, mice were sacrificed and tumors were excised and weighed. Inhibition of tumor growth was determined using the formula: (Tumor weight of control group -Tumor weight of treatment group)/tumor weight of control group × 100%.
Detection of expression of endostatin in vivo
Lung tissues were harvested after sacrificing the mice and the expression of endostatin was assayed by immunohistochemistry. They were then fixed in 40 g·L-1 paraformaldehyde and frozen. The sections of 20 mm were cut and mounted on glass slides. The sections were subsequently treated with 0.1 mol·L-1 PBS (pH7.0) for 5 min, and methanol mixed with 3 mL·L-1 H2O2 for 10 min. The sections were blocked with 10 g·L-1 milk in PBS, incubated at 37 °C for 2 h with a 1:1000 dilution of a monoclonal mouse anti-HA antibody, followed by 1 h incubation at 37 °C of 1:25 dilutions of goat-anti-mouse IgG-HRP. The sections were stained with 0.5 mL·L-1 DAB mixed with 0.3 mL·L-1 H2O2.
Immunohistochemistry
Mice were sacrificed after 18 d of treatment. Tumors were excised and fixed in 40 g·L-1 paraformaldehyde. Tumor tissues were frozen, and 20 μm sections were cut and mounted on glass slides. Sections were treated as described above. The primary CD-31 monoclonal rat anti-mouse antibody (Pharmingen Co) was diluted 200 times. The secondary antibody was a 1:25 dilution of goat anti-rat IgG-HRP (Kirkegaard & Perry Laboratories Co). Sections were stained with DAB as described above.
DNA fragmentation assay
The tumor tissues were excised and homogenized thoroughly. Following centrifugation at 3000 × g at 4 °C for 5 min, the pellets were resuspended in a lysis buffer containing 10 mmol·L-1 Tris-HCl buffer (pH8.0), 10 mmol·L-1 EDTA, 10 g·L-1 SDS, 20 mg·L-1 DNase-free RNase and 200 mg·L-1 of proteinase K. After overnight incubation at 37 °C, the DNA was purified by phenol/chloroform extraction, precipitated, and resuspended in TE buffer (pH7.4). Ten mg DNA was subjected to electrophoresis on a 15 g·L-1 agarose gel containing 0.5 mg·L-1 ethidium bromide and visualized under UV light. Electrophoresis was carried out in TE buffer (pH8.0) at 20 V for 8 h.
RESULTS
Detection of liposome diameter and encapsulation coefficiency of the TF-liposome-plasmid complex
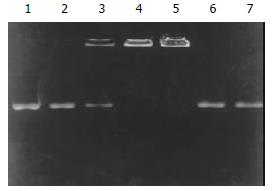
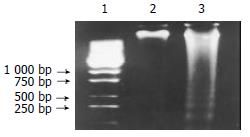
Prepared liposomes were observed under scanning electron microscopy at 10000 fold magnification. The mean diameter of the liposomes was about 180-220 nm. After formation of the TF-liposome-pcDNA3.0/endostatin complex, the encapsulation coefficiency was determined by 7.5 g·L-1 agarose gel electrophoresis (Figure 1). The results showed that the encapsulation coefficiency rose with increased ratio of liposome:DNA. Moreover, at a TF:liposome:DNA ratio of 10:10:1 (mg/nmol/mg), the encapsulation coefficiency was higher than 98%.
Figure 1 Determination of encapsulation coefficiency.
Lane 1: pure pcDNA3.0/endostatin plasmid, lanes 2-5: TF:liposome:DNA (mg:nmol:mg) at a ratio of 10:1:1, 10:5:1, 10:10:1 and 10:20:1. Lanes 6-7: complexes dissociated with 4 g·L-1 SDS.
Expression of endostatin in vitro and in vivo
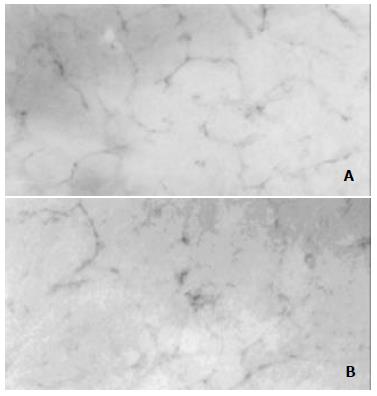
The in vitro expression of endostatin was detected by Western blot analysis. Distinct bands at around 22 kDa, the predicted molecular weight for the recombinant endostatin, were visualized in the supernatant and HUVEC cells transfected with pcDNA3.0/endostatin but not in the supernatant or HUVEC cells transfected with pcDNA3.0 (Figure 2). The in vivo expression of endostatin was detected by immunohistochemistry. After five administrations of the TF-liposome-pcDNA3.0/endostatin complex, the lung tissue was harvested and sectioned. Staining with an anti-HA antibody revealed positive cells containing endostatin in the sections of the treated group, whereas control sections were negative (Figure 3).
Figure 2 Expression of endostatin in vitro.
Lane 1: HUVEC cells transfected with pcDNA3.0/endostatin; lane 2: supernatant of HUVEC cells transfected with pcDNA3.0/endostatin; lane 3: HUVEC cells transfected with pcDNA3.0; lane 4: supernatant of HUVEC cells transfected with pcDNA3.0.
Figure 3 Expression of pcDNA3.
0/endostatin in vivo. The lung tissue sections from mice treated with TF-liposome-pcDNA3.0/endostatin (A) and from mice treated with TF-liposome- pcDNA3.0 (B) were treated with anti-HA antibody.
Endostatin inhibits the growth of primary liver tumors
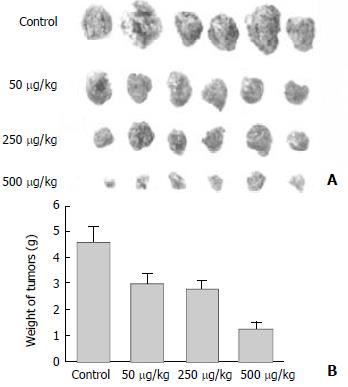
To determine the efficacy of the TF-liposome-endostatin complex on tumor growth, we treated tumor-bearing mice with aerosol containing the complex. Tumor growth in the mice treated with 50, 250 and 500 mg DNA/kg was inhibited by 36.6%, 40.8%, and 72.8%, respectively (P < 0.01). We concluded that the growth of primary tumors was significantly suppressed by systemic therapy with TF-liposome-pcDNA3.0/endostatin in a dose-dependent manner (Figure 4).
Figure 4 TF-liposome containing endostatin inhibits tumors growth.
A: Tumors excised from mice treated with different doses of TF-liposome-pcDNA3.0/endostatin, or with TF-liposome-pcDNA3.0 (negative control). B: (PDF) Tumor weight of mice treated with TF-liposome-pcDNA3.0/endostatin or TF-liposome-pcDNA3.0 vector. Each bar represents the mean ± SD for six mice.
Endostatin inhibits angiogenesis in primary tumors
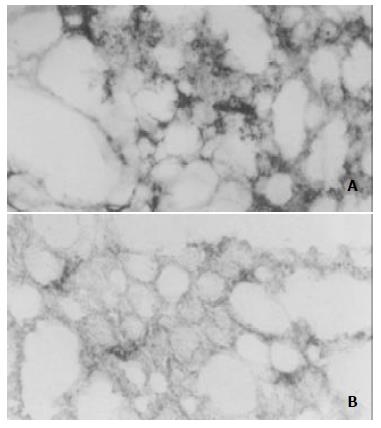
Immunohistochemical analysis showed a potent inhibition of angiogenesis in the tumors treated with the TF-liposome-pcDNA3.0/endostatin complex. Blood vessels were counted in three areas of each tumor section. There were significantly (P < 0.01) less microvessels in the tumors of treated mice versus control mice after staining of the tumor tissues with a rat anti-mouse CD-31 monoclonal antibody (Figure 5).
Figure 5 Reduced angiogenesis in TF-liposome-pcDNA3.
0/endostatin treated tumors. Tumor sections from mice treated with TF-liposome-pcDNA3.0 (A) or treated with TF-liposome-pcDNA3.0/endostatin (B) were stained with a CD-31 monoclonal antibody. CD-31-positive cells were brown whereas negative cells colorless in stain.
Quantitation of vessel density was determined under microscopicopy of the areas of brown (positive) and colorless (negative) staining at 432-fold magnification. One unit visual field encompassed 128 × 128 pixels. The positively stained area of the endostatin-treated group was significantly lower (P < 0.01, 645.8 ± 55.2 μm2, mean ± SD) than that of the control group (1325.4 ± 198.5 μm2, mean ± SD), which showed that the TF-liposome-pcDNA3.0/endostatin complex blocked tumor angiogenesis, while the TF-liposome-pcDNA3.0 vector complex, as a negative control, did not block angiogenesis, which indicated that the inhibition of angiogenesis was caused by the introduced endostatin gene.
Induction of apoptosis in tumor cells by introduced endostatin gene
Fragmentation of cellular DNA represents a main change in the nuclei of cells undergoing apoptosis. Administration of the TF-liposome-pcDNA3.0/endostatin complex resulted in the typical DNA ladder pattern in apoptotic cells of tumor tissues (Figure 6). However, no DNA fragmentation could be detected in the tumor tissues from mice treated with the empty vector complex. The results showed that the introduced endostatin gene blocked angiogenesis accompanied by induction of apoptosis in liver tumors.
Figure 6 The introduced endostatin gene induces apoptosis in cells of tumor tissues.
DNA was extracted from tumors and DNA fragmentation identified on a 15 g·L-1 agarose gel. Lane 1: the DNA molecular weight marker; lane 2: DNA from mice treated with TF-liposome-pcDNA3.0 vector; lane 3: DNA from mice treated with TF-liposome-pcDNA3.0/endostatin.
DISCUSSION
Inhibition of angiogenesis has recently been acknowledged as a promising strategy to treat cancer. Endostatin, an endogenous angiogenesis inhibitor, specifically suppresses endothelial cell proliferation, thereby acting as a competitor for angiogenesis inducers secreted by tumor cells. The use of endostatin may serve as an attractive new strategy in cancer therapy[22-25]. However, poor solubility of the endostatin protein together with a high effective dose hampered its widespread application, although high and continuous endostatin expression can be obtained by direct induction of the endostatin gene in vivo.
In recent years, great progress has been made in anti-cancer gene therapy and different gene delivery systems, based on retroviruses[15,26,27], adenoviruses[14,28] and liposomes have been used in animals. Liposomes are considered to provide a safer gene therapy delivery method, and can be administered intravenously, intramuscularly, intraperitoneally, or by intratracheal injection[17,18,20,29]. We used a transferrin facilitated liposome delivery of the endostatin gene by aerosol administration in our experiments. This method provides several advantages: (1) Lung tissue and airways cover a large surface area, therefore the introduced gene can be expressed efficiently in the lung; (2) TF enhances the efficiency of gene expression and transfection by facilitating the entry of DNA into the cells; (3) Recent liposome toxicity studies in the lungs of humans and several animal species show that liposomes cause no or minimal host inflammatory and immune responses[30,31]; (4) Aerosol administration is convenient and can be performed repeatedly by the patients themselves.
In the TF-liposome-DNA mediated gene transfer system, transferrin facilitated the delivery of DNA by binding to its receptors. The liposomes along with the DNA were internalized by fusion with the plasma membrane and underwent receptor-mediated endocytosis. Following the internalization of the TF-liposome-DNA-TF receptor complex, transferrin may facilitate the escape of DNA from the endosome. So the transfection efficiency was improved by this transfer system[18,32]. Indeed, the degree of inhibition of tumor growth (72.8%) in our studies was greater than that reported by another group (49%)[17], who used liposomes alone to deliver the endostatin gene.
The potent antiangiogenic effect of endostatin can specifically inhibit the proliferation and migration of endothelial cells and subsequently promote the development of apoptosis and atrophy of tumors without direct influence on tumor cell or nonneoplastic cell growth[33-35]. Our results demonstrated that typical DNA fragmentation was found in the nuclei of cells from tumor tissues of the mice treated with TF-liposome-pcDNA3.0/endostatin complexes, but not in the control group.
In conclusion, our results showed a strong inhibition of angiogenesis and tumor growth in mice after administration of the TF-liposome-endostatin plasmid complex. This suggests that TF-liposome-DNA complexes can be utilized as an efficient method for anti-angiogenic gene delivery to tumors in the gene therapy for cancers.