Published online Feb 15, 2003. doi: 10.3748/wjg.v9.i2.250
Revised: August 24, 2002
Accepted: September 5, 2002
Published online: February 15, 2003
AIM: To study the expression of cyclooxygenase-2 (COX-2) gene in gastric cancer and the relationship between COX-2 expression and clinicopathologic features of gastric cancer.
METHODS: With reference to the expression of β-actin gene, COX-2 mRNA level was examined in cancerous tissues and adjacent noncancerous mucosa from 33 patients by semiquantitative reverse transcription- polymerase chain reaction (RT-PCR). Quantitation of relative band Adj volume counts was performed using molecular Analyst for windows software. The COX-2 index was determined from the band Adj volume counts ratio of COX-2 to constitutively expressed actin.
RESULTS: The COX-2 index in gastric carcinoma was significantly higher than that in normal mucosa (0.5966 ± 0.2659 vs 0.2979 ± 0.171, u = 5.4309, P < 0.01). Significantly higher expression of COX-2 mRNA was also observed in patients with lymph node involvement than that in those without (0.6775 ± 0.2486 vs 0.4105 ± 0.2182, t = 2.9341, P < 0.01). Furthermore, the staging in the UICC TNM classification significantly correlated with COX-2 overexpression (F = 3.656, P < 0.05), the COX-2 index in stage III and IV was significantly higher than those in stage I and II (q = 3.2728 and q = 3.4906, P < 0.05). The COX-2 index showed no correlation with patient抯 age, sex, blood group, tumor location, gross typing, depth of invasion, differentiation, and the greatest tumor dimension (P > 0.05).
CONCLUSION: Expression of COX-2 mRNA in gastric carcinoma was significantly higher, which may enhance lymphatic metastasis in patients with gastric carcinoma. The staging in the UICC TNM classification was significantly correlated with COX-2 over-expression. COX-2 may contribute to progression of tumor in human gastric adenocarcinoma.
- Citation: Xue YW, Zhang QF, Zhu ZB, Wang Q, Fu SB. Expression of cyclooxygenase-2 and clinicopathologic features in human gastric adenocarcinoma. World J Gastroenterol 2003; 9(2): 250-253
- URL: https://www.wjgnet.com/1007-9327/full/v9/i2/250.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i2.250
Cyclooxygenase-2 (COX-2) is a rate-limiting enzyme in conversion of arachidonic acid to prostaglandins, also referred to as prostaglandin endoperoxide synthase (PGHS). Epidemiological and clinical results have suggested that non-steroidal anti-inflammatory drugs (NSAIDs) may reduce the risk of digestive tract carcinomas, including gastric and colon lesions. COX-2, which has been identified as being overexpressed in colorectal and gastric cancers, is one of the major targets of NSAIDs. Not only specific inhibitors of COX-2 significantly suppressed proliferation of gastric cancer cell lines[1,2], but also a recent in-vitro study suggested that specific inhibitors of COX-2 significantly suppressed proliferation of gastric cancer xenografts in nude mice[3]. This evidence supports the hypothesis that COX-2 is an important factor in the growth and development of gastric cancer. We used the semiquantitative reverse transcription- polymerase chain reaction (RT-PCR) to examine the expression of COX-2 mRNA in gastric cancerous tissues and adjacent noncancerous mucosa, and to study the relationship to the clinicopathologic features.
Thirty-three patients undergoing surgery for primary gastric cancer at the Third Clinical Hospital of Harbin Medical University from 2001 to 2002 were examined. Of these, 27 were male and 6 were female. The mean age was 58.6 years (range, 32-76). The clinicopathologic features showed in Table 1. Paired samples of cancer tissue and normal gastric mucosa (the distance to border of tumor is beyond 5 cm) were obtained from each patient at the time of surgery. The samples were immediately frozen in liquid nitrogen. All specimens were verified by the same pathologists.
| Parameter | -x ± s | P |
| Sex | ||
| male (n = 27) | 0.5798 ± 0.2754 | NSa |
| female (n = 6) | 0.6722 ± 0.2012 | |
| Age (range) | ||
| ≤ 60 (n = 16) | 0.6144 ± 0.2655 | NS |
| > 60 (n = 17) | 0.5799 ± 0.2651 | |
| Blood group | ||
| A type (n = 10) | 0.5928 ± 0.2647 | NS |
| B type (n = 11) | 0.6415 ± 0.264 | |
| O type (n = 7) | 0.5906 ± 0.2614 | |
| AB type (n = 5) | 0.6221 ± 0.2551 | |
| Greatest tumor dimension | ||
| < 5 cm (n = 11) | 0.4929 ± 0.2807 | NS |
| ≥ 5 cm (n = 22) | 0.6485 ± 0.242 | |
| Histopathologic type | ||
| Middling differentiation (n = 7) | 0.5772 ± 0.2645 | NS |
| Low differentiation (n = 9) | 0.5563 ± 0.249 | |
| Middling and low differentiation (n = 6) | 0.4278 ± 0.2911 | |
| Signet-ring/mucous cell (n = 11) | 0.7341 ± 0.186 | |
| Gross type | ||
| Borrmann I (n = 8) | 0.4897 ± 0.28 | NS |
| Borrmann II III (n = 22) | 0.6244 ± 0.2672 | |
| Borrmann IV (n = 3) | 0.6785 ± 0.0443 | |
| Depth of invasionb | ||
| pT2 (n = 10) | 0.5198 ± 0.2516 | NS |
| pT3 (n = 11) | 0.5682 ± 0.2526 | |
| pT4 (n = 12) | 0.686 ± 0.2633 | |
| Lymph nodeb | ||
| pN0 (n = 10) | 0.4105 ± 0.2182 | < 0.05 |
| pN1 and pN2 (n = 23) | 0.6775 ± 0.2486 | |
| TNM stageb | ||
| I and II (n = 9) | 0.4054 ± 0.2275 | < 0.05 |
| III (n = 13) | 0.6561 ± 0.2233 | |
| IV (n = 11) | 0.6829 ± 0.2631 | |
| Tumor location | ||
| Antrum (n = 18) | 0.5678 ± 0.314 | NS |
| Corpus (n = 6) | 0.6771 ± 0.1488 | |
| Cardiac orifice (n = 9) | 0.6006 ± 0.203 |
Total RNA was isolated from 50-100 mg of the tissues according to the method of User Manual TRIzol reagent (Life Science) and was quantitated by reading absorbance at 260 nm. The total RNA solution was subjected to RT-PCR analysis using the TITANIUMTM one-step RT-PCR kit (CLONTECH Laboratories, Inc, USA). The total RNA specimens (1 μg) were reverse transcripted and amplified in 25 μL of reaction mixture, and the reaction conditions for COX-2 and β-actin was identical.
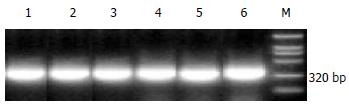
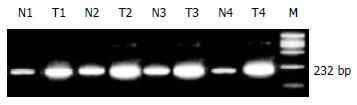
Oligonucleotide primers for COX-2 used were 5'-TGA AAC CCA CTC CAA ACA CAG-3' (sense) and 5'-TCA TCA GGC ACA GGA GGA AG-3' (antisense), the PCR product length was 232 bp; those for β-actin were 5'-GTT TGA GAC CTT CAA CAC CCC-3' (sense) and 5'-GTG GCC ATC TCT CTT GCT CGA AGT C-3' (antisense), the PCR product length was 320 bp. The RT-PCR procedures were as following: reverse transcription at 55 °C for 60 min, inactivation of reverse transcriptase at 94 °C for 5 min, amplification for 30 cycles of denaturation at 94 °C for 30 sec, annealing at 55 °C for 30 sec, and extension at 68 °C for 60 sec.
The PCR products were electrophoresed in 2% agarose gels with 0.5 μg/mL ethidium bromide and visualized under UV light. Quantitation of relative band volume counts was performed using Molecular Analyst for windows software. To estimate COX-2 expression levels, a COX-2 index was designated as a band volume counts ratio of COX-2 to constitutively expressed β-actin, because β-actin mRNA is expressed constitutively in both the normal gastric mucosa and tumor tissues. The higher COX-2 index showed the higher expression level of COX-2 mRNA in tissues.
Statistical significance was calculated with the Students t test, Students u test, one-way ANOVA and Student-Newman-Keuls. P < 0.05 was selected as the statistically significant value. All results are shown as means ± SE.
The total RNA was electrophoresed in 1% agarose gels with 0.5 μg/mL ethidium bromide and visualized under UV light, then showed three bands: 5 s, 18 s and 28 s. The total RNA was quantitated by reading absorbance at 260 nm, and A260/A280 that ranged from 1.7 to 2.0.
COX-2 mRNA and β-actin mRNA were amplified by RT-PCR, and their products length were 232bp and 320bp. The β-actin mRNA expressed constitutively in all tissues, including normal gastric mucosa and tumor tissues (Figure 1). COX-2 mRNA expressed in 29 of 33 (87.88%) human gastric cancer specimens, over-expression was seen in 26 of 33 (78.79%) cases (COX-2 index was 0.5966 ± 0.2659), and weak or negative COX-2 expression was seen in 25 of 33 (75.8%) normal mucosa (COX-2 index was 0.2979 ± 0.171) (Figure 2). COX-2 index in gastric carcinoma was significantly higher than that in normal mucosa (u = 5.4309, P < 0.01). Significantly higher expression of COX-2 mRNA was also observed in patients with lymph node involvement than that in those without (0.6775 ± 0.2486 vs 0.4105 ± 0.2182, t = 2.9341, P < 0.01). Furthermore, the staging in the UICC TNM classification (1985) significantly correlated with COX-2 overexpression (F = 3.656, P < 0.05), COX-2 index in stages III and IV was significantly higher than those in stages I and II (q = 3.2728 and q = 3.4906, P < 0.05). COX-2 index showed no correlation with patient's age, sex, blood group, tumor location, gross typing, depth of invasion, differentiation, and the greatest tumor dimension (P > 0.05, Table 1).
Two isoforms of COX have been identified: COX-1 expressed constitutively in a number of cell types, which take part in sustaining physiologic function of body; COX-2 is a inducing immediate-early gene, and human gastric mucosa normally expresses detectable levels of COX-2. COX-2 is induced by a variety of cytokines, hormones, and tumor promoters, leading to more PGs producing. Initial association of COX-2 with tumor has shown in studies for colorectal cancer, and then for other tumor[4-10]. A series of studies confirmed that COX-2 levels elevated in colorectal carcinoma, and over-expression of COX-2 in colorectal cancer was associated with carcinogenesis, develop ment[11-17] and poor prognosis[18,19]. In recent years, a lot of researchers studied the association of COX-2 expression with gastric carcinoma[20-26], but those conclusions were not identical, with a variety of causes.
In the current study, we found that elevated levels of COX-2 mRNA in human gastric adenocarcinoma tissues, and the COX-2 index in gastric carcinoma was significantly higher than that in normal mucosa (u = 5.4309, P < 0.01), which is identical to the data published[20-26]. The mechanism of the COX-2 involved in the pathogenesis of tumor is that over-expression of COX-2 may promote PGs biosynthesis in gastric cancer cells, and PGs shows a potent immunosuppression effect by inhibiting the T-cell or natural killer cell activity[27]. PGs thus provide a selective advantage for cancer cell survival; in addition, COX-2 can also suppress cell apoptosis[28-32], prolong cell cycle G1[33,34], and decrease level of cyclin D1[35,36], which lead to that cells can not enter the cycle and proliferate continuously. Meantime, COX-2 enhances adhesion of cells[37,38] and promotes tumor angiogenesis[22,39], which may finally lead to the carcinogenesis and progression of the tumor.
Many researchers[20-40] found that COX-2 mRNA expression in gastric carcinoma is correlated closely with depth of invasion, indicating that COX-2 is involved in the growth of the tumor. But, our studies did not find a correlation between COX-2 expression and the extent of primary tumor invasion. Our recent studies have found a correlation between the level of COX-2 expression and lymph node metastasis in patients with gastric carcinoma. Murata et al[21] and Leung et al[41] found that tumor with the overexpression of COX-2 protein by Western blot and immunohistochemical analysis was associated significantly with invasion into gastric wall lymphatic vessels as well as with metastasis to lymph nodes (P < 0.05). Uefuji et al[40] detected level of COX-2 mRNA expression by RT-PCR analysis, and the conclusion was in agreement with this study. We demonstrated that significantly higher expression of COX-2 mRNA was also observed in patients with lymph node involvement than that in those without (t = 2.9341, P < 0.01). It is suggested that COX-2 may influence lymphatic involvement by the way of increasing tumor invasiveness. Some studies have found that over-expression of COX-2 decreased the expression of both E-cadherin and the transforming growth factor-b receptor, which has been linked to enhancing tumorigenic potential and increasing tumor invasiveness[37,38,42-45]. Meantime, the overexpression of the COX-2 promotes invasiveness in gastric cancer through the induction of metalloproteinase-2 and membrane-type metalloproteinase[11,21,46].
Although several investigators[20] reported that the COX-2 level was not associated with UICC TNM stage, but majority of them[21,23] have reported a significant relation between the levels of COX-2 protein over-expression and UICC TNM stage. Our results are in agreement with previous ones. The COX-2 level in Stage III and IV was significantly higher than in Stage I and II (q = 3.2728 and q = 3.4906, P < 0.05); but the difference of COX-2 level between Stages III and IV showed no statistical significance (q = 0.3702, P > 0.05). Consequently, we can infer that COX-2 may be independent or synergistic with other factors to promote growth of gastric cancer, and to enhance the lymph node metastasis and involvement. But, in advanced gastric cancer, when COX-2 expression is up-regulated to a certain level, COX-2 can not increase continuously, which suggests that there are some much more complicated mechanisms in the regulation of COX-2 expression.
In conclusion, COX-2 mRNA shows elevated expression in gastric carcinoma tissues, and the degree of COX-2 mRNA elevation is related to the lymphatic metastasis, UICC TNM stage, and poor prognosis. These findings suggest that COX-2 is involved in the carcinogenesis and growth of gastric carcinoma and that the inhibition of COX-2 activity may prove to have an important therapeutic benefit in the control of gastric carcinoma.
Edited by Zhang JZ
| 1. | Sawaoka H, Kawano S, Tsuji S, Tsujii M, Murata H, Hori M. Effects of NSAIDs on proliferation of gastric cancer cells in vitro: possible implication of cyclooxygenase-2 in cancer development. J Clin Gastroenterol. 1998;27 Suppl 1:S47-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Uefuji K, Ichikura T, Shinomiya N, Mochizuki H. Induction of apoptosis by JTE-522, a specific cyclooxygenase-2 inhibitor, in human gastric cancer cell lines. Anticancer Res. 2000;20:4279-4284. [PubMed] |
| 3. | Sawaoka H, Kawano S, Tsuji S, Tsujii M, Gunawan ES, Takei Y, Nagano K, Hori M. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol. 1998;274:G1061-G1067. [PubMed] |
| 4. | Wu QM, Li SB, Wang Q, Wang SH, Li XB, Liu CZ. The expression of COX-2 in esophageal carcinoma and its relation to clinicopathologic characteristics. Shijie Huaren Xiaohua Zazhi. 2001;9:11-14. |
| 5. | Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991-994. [PubMed] |
| 6. | Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987-990. [PubMed] |
| 7. | Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204. [PubMed] |
| 8. | Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimäki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997-5001. [PubMed] |
| 9. | Jang BC, Sanchez T, Schaefers HJ, Trifan OC, Liu CH, Creminon C, Huang CK, Hla T. Serum withdrawal-induced post-transcriptional stabilization of cyclooxygenase-2 mRNA in MDA-MB-231 mammary carcinoma cells requires the activity of the p38 stress-activated protein kinase. J Biol Chem. 2000;275:39507-39515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Liu XL, Fan DM. Protective effects of prostaglandin E1 on hepatocytes. World J Gastroenterol. 2000;6:326-329. [PubMed] |
| 11. | Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, Yang WK. Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int J Cancer. 2001;91:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165-170. [PubMed] |
| 13. | Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733-1740. [PubMed] |
| 14. | Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040-5044. [PubMed] |
| 15. | Cianchi F, Cortesini C, Bechi P, Fantappiè O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121:1339-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625-631. [PubMed] |
| 17. | Shen ZX, Cao G, Sun J. Expression of COX-2 mRNA in colorectal carcinomas. Shijie Huaren Xiaohua Zazhi. 2001;9:1082-1084. |
| 18. | Sheehan KM, Sheahan K, O'Donoghue DP, MacSweeney F, Conroy RM, Fitzgerald DJ, Murray FE. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999;282:1254-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Fosslien E. Review: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann Clin Lab Sci. 2001;31:325-348. [PubMed] |
| 20. | Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 21. | Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Uefuji K, Ichikura T, Mochizuki H. Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer. Clin Cancer Res. 2000;6:135-138. [PubMed] |
| 23. | Yamamoto H, Itoh F, Fukushima H, Hinoda Y, Imai K. Overexpression of cyclooxygenase-2 protein is less frequent in gastric cancers with microsatellite instability. Int J Cancer. 1999;84:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 24. | Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519-525. [PubMed] |
| 25. | Saukkonen K, Nieminen O, van Rees B, Vilkki S, Härkönen M, Juhola M, Mecklin JP, Sipponen P, Ristimäki A. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res. 2001;7:1923-1931. [PubMed] |
| 26. | Gao HJ, Yu LZ, Sun L, Miao K, Bai JF, Zhang XY, Lu XZ, Zhao ZQ. Expression of COX-2 protein in gastric cancer tissue and accompanying tissue. Shijie Huaren Xiaohua Zazhi. 2000;8:578-579. |
| 27. | Kojima M, Morisaki T, Uchiyama A, Doi F, Mibu R, Katano M, Tanaka M. Association of enhanced cyclooxygenase-2 expression with possible local immunosuppression in human colorectal carcinomas. Ann Surg Oncol. 2001;8:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Hsueh CT, Chiu CF, Kelsen DP, Schwartz GK. Selective inhibition of cyclooxygenase-2 enhances mitomycin-C-induced apoptosis. Cancer Chemother Pharmacol. 2000;45:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Murakami A, Takahashi D, Kinoshita T, Koshimizu K, Kim HW, Yoshihiro A, Nakamura Y, Jiwajinda S, Terao J, Ohigashi H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the alpha,beta-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Geloso MC, Vercelli A, Corvino V, Repici M, Boca M, Haglid K, Zelano G, Michetti F. Cyclooxygenase-2 and caspase 3 expression in trimethyltin-induced apoptosis in the mouse hippocampus. Exp Neurol. 2002;175:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Waskewich C, Blumenthal RD, Li H, Stein R, Goldenberg DM, Burton J. Celecoxib exhibits the greatest potency amongst cyclooxygenase (COX) inhibitors for growth inhibition of COX-2-negative hematopoietic and epithelial cell lines. Cancer Res. 2002;62:2029-2033. [PubMed] |
| 32. | Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483-487. [PubMed] |
| 33. | Trifan OC, Smith RM, Thompson BD, Hla T. Overexpression of cyclooxygenase-2 induces cell cycle arrest. Evidence for a prostaglandin-independent mechanism. J Biol Chem. 1999;274:34141-34147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Song S, Xu XC. Effect of benzo[a]pyrene diol epoxide on expression of retinoic acid receptor-beta in immortalized esophageal epithelial cells and esophageal cancer cells. Biochem Biophys Res Commun. 2001;281:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Dihlmann S, Siermann A, von Knebel Doeberitz M. The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene. 2001;20:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Bissonnette M, Khare S, von Lintig FC, Wali RK, Nguyen L, Zhang Y, Hart J, Skarosi S, Varki N, Boss GR. Mutational and nonmutational activation of p21ras in rat colonic azoxymethane-induced tumors: Effects on mitogen-activated protein kinase, cyclooxygenase-2, and cyclin D1. Cancer Res. 2000;60:4602-4609. [PubMed] |
| 37. | Zhang Z, DuBois RN. Detection of differentially expressed genes in human colon carcinoma cells treated with a selective COX-2 inhibitor. Oncogene. 2001;20:4450-4456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Rowland RG. Macroscopic, microscopic and molecular observations of bladder cancer. J Urol. 2001;165:1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM. Cyclooxygenase 2- and prostaglandin E (2) receptor EP (2)-dependent angiogenesis in Apc (Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506-511. [PubMed] |
| 40. | Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol. 2001;76:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 41. | Leung WK, To KF, Ng YP, Lee TL, Lau JY, Chan FK, Ng EK, Chung SC, Sung JJ. Association between cyclo-oxygenase-2 overexpression and missense p53 mutations in gastric cancer. Br J Cancer. 2001;84:335-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Sheng H, Shao J, O'Mahony CA, Lamps L, Albo D, Isakson PC, Berger DH, DuBois RN, Beauchamp RD. Transformation of intestinal epithelial cells by chronic TGF-beta1 treatment results in downregulation of the type II TGF-beta receptor and induction of cyclooxygenase-2. Oncogene. 1999;18:855-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Saha D, Datta PK, Sheng H, Morrow JD, Wada M, Moses HL, Beauchamp RD. Synergistic induction of cyclooxygenase-2 by transforming growth factor-beta1 and epidermal growth factor inhibits apoptosis in epithelial cells. Neoplasia. 1999;1:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | O'Mahony CA, Beauchamp RD, Albo D, Tsujii M, Sheng HM, Shao J, Dubois RN, Berger DH. Cyclooxygenase-2 alters transforming growth factor-beta 1 response during intestinal tumorigenesis. Surgery. 1999;126:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Shao J, Sheng H, Aramandla R, Pereira MA, Lubet RA, Hawk E, Grogan L, Kirsch IR, Washington MK, Beauchamp RD. Coordinate regulation of cyclooxygenase-2 and TGF-beta1 in replication error-positive colon cancer and azoxymethane-induced rat colonic tumors. Carcinogenesis. 1999;20:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Ji F, Wang WL, Yang ZL, Li YM, Huang HD, Chen WD. Study on the expression of matrix metallo proteinase-2 mRNA in human gastric cancer. World J Gastroenterol. 1999;5:455-457. [PubMed] |