Published online Dec 15, 2003. doi: 10.3748/wjg.v9.i12.2828
Revised: May 22, 2003
Accepted: June 12, 2003
Published online: December 15, 2003
AIM: To identify the expression of Caspase-1(interleukin-1β converting enzyme) and its role in adenoma of the pancreas and chronic pancreatitis.
METHODS: The expression of Caspase-1 was assessed in 42 pancreatic cancer tissue samples, 38 chronic pancreatitis specimens, and 9 normal pancreatic tissues by immunohistochemistry and Western blot analysis.
RESULTS: Overexpression of Caspase-1 was observed in both disorders, but there were differences in the expression patterns in distinct morphologic compartments. Pancreatic cancer tissues showed a clear cytoplasmatic overexpression of Caspase-1 in tumor cells of 71% of the tumors, whereas normal pancreatic tissues showed only occasional immunoreactivity. In chronic pancreatitis, overexpression of Caspase-1 was found in atrophic acinar cells (89%), hyperplastic ducts (87%), and dedifferentiating acinar cells (84%). Although in atrophic cells a clear nuclear expression was found, hyperplastic ducts and dedifferentiating acinar cells showed clear cytoplasmic expression. Western blot analysis revealed a marked expression of the 45 kDa precursor of Caspase-1 in pancreatic cancer and chronic pancreatitis (80% and 86%, respectively). Clear bands at 30 kDa, which suggested the p10-p20 heterodimer of active Caspase-1, were found in 60% of the cancer tissue and 14% of the pancreatitis tissue specimens, but not in normal pancreatic tissues.
CONCLUSION: Overexpression of Caspase-1 is a frequent event in pancreatic disorders and its differential expression patterns may reflect two functions of the protease. One is its participation in the apoptotic pathway in atrophic acinar cells and tumor-surrounding pancreatitis tissue, the other is its possible role in proliferative processes in pancreatic cancer cells and hyperplastic duct cells and dedifferentiating acinar cells in chronic pancreatitis.
- Citation: Yang YM, Ramadani M, Huang YT. Overexpression of Caspase-1 in adenocarcinoma of pancreas and chronic pancreatitis. World J Gastroenterol 2003; 9(12): 2828-2831
- URL: https://www.wjgnet.com/1007-9327/full/v9/i12/2828.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i12.2828
Caspase-1 was the first described member of a group of cysteine proteases called Caspases. It was formerly designated as interleukin-1β converting enzyme, and was originally characterized by its ability to cleave the inactive precursor of interleukin-1β to generate the active 17.5 kDa proinflammatory cytokine IL-1β[1]. It has been found to express in many tissues as an inactive 45 kDa precursor protein (p45) from which the active enzyme is generated by an autocatalytic cleavage process[2]. Caspase-1 was first isolated from human monocytic cell line THP 1. Because of its similarity in sequence to the death gene product CED-3 of nematode Caenorhabditis elegans, it has been regarded as a key enzyme of the apoptotic pathway[3]. Today, more than 10 Caspases have been identified and their roles in apoptosis are well known[4-6].
Several new members of the group of Caspases have been identified and described. Similar to Caspase-1, overexpression of any of these enzymes would lead to apoptosis in a variety of cell types[7-9]. We investigated the expression of apoptosis-related enzyme Caspase-1 in pancreatic cancer and chronic pancreatitis. Interestingly, we found a clear overexpression of Caspase-1 in pancreatic cancer tissue as well as in chronic pancreatitis specimens.
Pancreatic tissue samples were obtained from 42 patients with pancreatic cancer and 38 patients with chronic pancreatitis who underwent surgery at the Department of General Surgery, University of Ulm. The median age of the patients with pancreatic cancer (20 women and 22 men) was 61.8 years (range 38 to 78 years). The group of patients with chronic pancreatitis was composed of 13 women and 25 men. The median age of this group was 52.2 years (range 22 to 73 years). The main indication for pancreatic head resection was long-lasting pain (36 of 38 patients) and obstruction of the common bile duct (19 of 38 patients). In all patients, duodenum-preserving pancreatic head resection was performed.
Tissues were collected after surgical removal, immediately snap-frozen in liquid nitrogen, and stored at -80 °C, or fixed in 4% formalin for 24 hours at room temperature, processed, and embedded in paraffin. All 42 pancreatic cancer tissue and 38 chronic pancreatitis tissue samples were used for immunohistochemical analysis. Five normal pancreatic tissue samples from organ donors and four normal pancreatic tissue samples from patients undergoing surgery for pancreatic cancer served as control specimens. Twenty pancreatic cancer tissues, 14 chronic pancreatitis tissues, and seven normal pancreatic tissues underwent Western blot analysis.
Paraffin-embedded tissues were cut into 5 μm-thick sections and adhered to silanized slides, deparaffinized, and hydrated. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol. The tissue sections were covered with 5% normal goat serum (DAKO, Glostrup, Denmark) in Tris-buffered saline for 60 minutes and incubated overnight with polyclonal rabbit antihuman caspase-1 antibody (Upstate Biotechnology, Lake Placid, NY, USA.) in a dilution of 1:100. For each case, a corresponding section was incubated in Tris-buffered saline without the primary antibody as a control for nonspecific staining. Further negative controls consisted of normal rabbit serum instead of specific antiserum. Biotinylated pig anti-rabbit secondary antibody was added for 45 minutes, followed by avidin-biotinylated peroxidase complex for an additional 45 minutes. Staining was achieved using 3,3’-diaminobenzidine. The sections were then counterstained with Mayer’s hemalum and mounted.
Immunohistochemical findings were scored depending on the extent and intensity of staining. Both intensity and extent were assessed in regions with hyperplastic ducts and atrophic acinar cells. All sections were graded by two experienced investigators who had no knowledge of the clinical data. At least 10 randomly selected high-power fields were scored. The intensity of staining was graded on a four points scale of 0 = no staining, 1 = weak, 2 = moderate, and 3 = strong. The extent of positive immunoreactivity was graded by the percentage of stained cells in the region of interest: 0 point = 0%, 1 point ≤ 20%, 2 points = 20%-50%, and 3 points ≥ 50%. An overall score was obtained by the product of intensity and extent of positive staining. Cases with 0 points were considered to be negative, cases with a final score of 1-3 were considered to be weakly positive, cases with a score of 4-7 were considered to be moderately positive, and cases with a final score greater than 7 were considered to be strongly positive.
Frozen pancreatic samples were finely diced with a surgical blade and washed twice with ice-cold phosphate-buffered saline. After swelling on ice for 60 minutes, the samples were dissociated by sonication. The lysates were centrifuged and the protein fraction was aliquoted and stored at -80 °C until further analysis. For immunoblotting, the lysates were boiled in sodium dodecyl sulfate-gel sample buffer for 5 minutes. Thirty micrograms of protein were electrophoretically resolved on denaturing 15% polyacrylamide gels with a 3% stacking gel. Proteins were transferred to nitrocellulose membranes using a transblot apparatus (Phase, Lubeck, Germany). Nonspecific interactions were blocked by preincubation of the membranes with a milk powder suspension overnight at 4 °C. After incubation of the membranes with monoclonal antibodies, the binding of antibodies was detected using the ECL-system (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The autocleavage experiments of the monocytic cell line THP1 were performed as previously described[10].
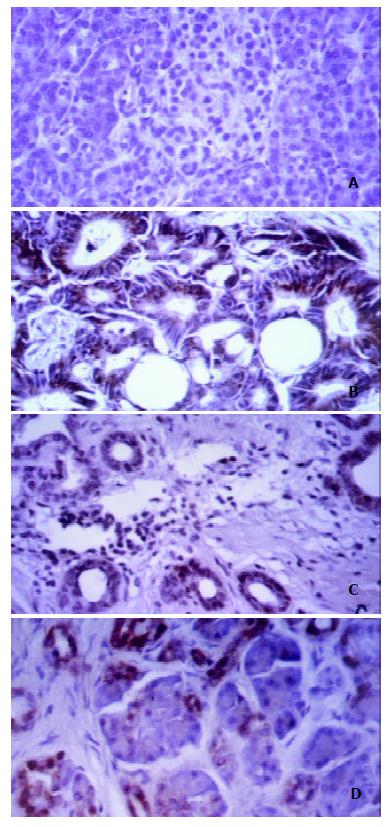
Staining of pancreatic tissue specimens with a polyclonal rabbit antiserum recognizing Caspase-1 revealed a marked overexpression of Caspase-1 in pancreatic cancer and chronic pancreatitis. Although normal pancreatic tissue showed only occasional slight staining (Figure 1A), we found predominantly cytoplasmic immunoreactivity of cancer cells in 71% of the pancreatic tumors (Figure 1B). In primary chronic pancreatitis tissue samples, Caspase-1 overexpression was found in atrophic acinar cells, hyperplastic ducts, and acinar cells that appeared to dedifferentiate to form tubular structures. Hyperplastic ducts showed clear cytoplasmic staining in 87% ( Figure 1C). Atrophic acinar cells with pyknotic nuclei were stained positive in 89% of the pancreatitis tissues, but the immunoreactivity was predominantly nuclear (Figure 1D). Dedifferentiating acinar cells showed positive cytoplasmic immunostaining with Caspase-1 antiserum in 84%. In chronic pancreatitis tissues, which often surrounded pancreatic carcinoma because of tumor obstruction, we also found strong Caspase-1 expression, but immunoreactivity differed from that of chronic pancreatitis tissue specimens from patients without malignancy. The staining in tumor-surrounding pancreatitis tissues was generally stronger than that in non-tumor-related pancreatitis tissues, and the distinct distribution pattern found in primary chronic pancreatitis could not be observed. In addition to this tissue-specific staining, a positive immunoreactivity of tissue-infiltrating lymphocytes was found in 73% of the tissues.
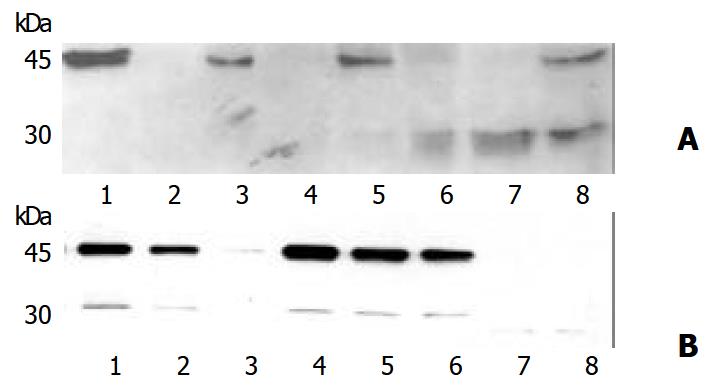
To confirm the overexpression of Caspase-1 seen in immunohistochemical staining, Western blot analysis was performed with monoclonal antibody CAL against human Caspase-1. This antibody was developed to detect the 20 kDa subunit of active Caspase-1[11], but also detect the p45 precursor. Pancreatic cancer tissue as well as chronic pancreatitis tissue specimens showed specific bands migrating at 45 kDa (Figure 2) which suggested the p45 precursor protein of Caspase-1. This band was found in 80% of cancer tissues and 86% of chronic pancreatitis tissues.
Lysates from THP-1 cells served as control specimens for active Caspase-1. In monocytic THP1 cells, p45 Caspase-1 precursor has been known to be cleaved by an autocatalytic process to the active Caspase-1 enzyme when kept at room temperature for 24 hours[11]. In 60% of the cancer probes and 14% of the pancreatitis lysates, and in lysates from the autocleavage experiments of THP1 cells, a further band at 30 kDa was detectable, which was suggested the active p10-p20 heterodimer of active Caspase-1. In pancreatic tissue of healthy organ donors, no signal was obtained using monoclonal antibody against human Caspase- 1, suggesting an overexpression of Caspase-1 protein in pancreatic cancer and chronic pancreatitis. Since lysates from pancreatic cancer tissue and chronic pancreatitis specimens also showed the 30 kDa band, it was plausible that Caspase-1 was at least partly activated in these disorders.
To assess the clinical importance of Caspase-1 overexpression in pancreatic cancer, we correlated the immunohistochemical findings with age, sex, tumor extent, lymph node metastasis, and grading. As a result, no correlation was found between Caspase-1 expression and any of these clinicopathologic features. In addition, no statistical difference was found with regard to postoperative survival. In patients with chronic pancreatitis, we correlated the expression of Caspase-1 with age, sex, onset of disease, need for analgesic drugs, and endocrine and exocrine pancreatic function. As in patients with pancreatic cancer, no correlation with any of the tested features could be found.
Caspases play an important role in the apoptotic pathway in a variety of cell types. However, little is known about the physiologic roles of different homologues during apoptosis. We assessed the expression of Caspase-1 in pancreatic cancer and chronic pancreatitis. Interestingly, immunohistochemical analysis revealed a clear overexpression of this enzyme in both disorders, but also differences in the expression patterns in distinct morphologic compartments. Furthermore, Western blot analysis of pancreatic cancer tissues and chronic pancreatitis tissues showed that Caspase-1 was at least partially activated in these diseases.
Caspase-1 is described as a cytosolic protein. However, in our experiments we found a clear nuclear staining with the antibody against human Caspase-1 in atrophic acinar cells in chronic pancreatitis specimens. Interestingly, most of the known substrates for Caspases in apoptosis were structural or catalytic nuclear proteins, the cleavage fragments of which were found in apoptotic bodies[12] . The nuclear immunoreactivity of atrophic acinar cells in chronic pancreatitis may, therefore, be an indication of ongoing apoptotic processes. In contrast, the marked cytoplasmatic overexpression of Caspase-1 in tumor cells could hardly be explained by apoptosis, since some tumors showed Caspase-1 overexpression in nearly all cancer cells. Furthermore, we found a clear correlation between Caspase-1 overexpression in pancreatic carcinoma and cyclin D1, which has been known to be involved in cellular proliferation and to contribute to an aggressive behavior in many tumors[13-16]. EGF and EGF-R have been shown to play a crucial role in autocrine stimulation of human pancreatic carcinoma[17]. In the pancreatic cancer tissues we investigated, the cytoplasmatic expression of Caspase-1 in pancreatic cancer cells also correlated significantly with the expression of EGF and EGF-R. Interestingly, it has recently been shown that EGF was also able to inhibit cell growth and induce apoptosis via Caspase-1 induction[18]. However, with regard to the fact that cyclin D1, EGF, and EGF-R overexpression was associated with poor prognosis in human pancreatic cancer[13,19], it is hard to believe that these factors could be an indication for the apoptotic state of these tumors.
Chronic pancreatitis is histologically characterized by the destruction of the pancreatic parenchyma, irregular sclerosis, and focal duct cell proliferation. Besides the predominantly nuclear staining with the antibody against Caspase-1 in atrophic acinar cells, we found a clear cytoplasmatic overexpression in two other distinct morphologic compartments in chronic pancreatitis—in hyperplastic ducts and in areas with tubularily dedifferentiating acinar cells. Cyclin D1, EGF, and EGF-R were also altered in chronic pancreatitis[20,21], which lend support to the hypothesis that chronic pancreatitis is a progressive process. Furthermore, we have recently found that positive nuclear MIB-1 (Ki67) expression in pancreatitis tissues might be an indication for proliferative processes[22,23]. Tumor surrounding pancreatitis tissues from patients with pancreatic cancer showed a strong positive immunoreactivity with antiserum against Caspase-1, but the differential expression pattern seen in primary chronic pancreatitis could not be observed. Nuclear and cytoplasmatic expression of Caspase-1 was found in atrophic acinar cells and in duct cells as well. One explanation for this overexpression might be the dramatic course of pancreatitis due to tumor obstruction. Another explanation might be that Caspase-1 was upregulated through paracrine stimulation with tumor-derived EGF in these cells. Nevertheless, destruction of pancreatic parenchyma due to tumor growth and invasion was likely to be associated with apoptosis of normal pancreatic cells.
In summary, Caspase-1 is overexpressed in atrophic acinar cells of chronic pancreatitis and tumor-surrounding pancreatitis tissues and is markedly expressed in cytoplasm of pancreatic cancer cells and hyperplastic duct cells and dedifferentiating acinar cells in chronic pancreatitis.
Edited by Ma JY
| 1. | Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci USA. 1989;86:5227-5231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 390] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 2. | Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1857] [Cited by in RCA: 1983] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 3. | Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1737] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 4. | Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 574] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 5. | Zhu H, Fearnhead HO, Cohen GM. An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells. FEBS Lett. 1995;374:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 121] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Jacobsen MD, Weil M, Raff MC. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 311] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Duan H, Chinnaiyan AM, Hudson PL, Wing JP, He WW, Dixit VM. ICE-LAP3, a novel mammalian homologue of the Caenorhabditis elegans cell death protein Ced-3 is activated during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1996;271:1621-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 234] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Faucheu C, Diu A, Chan AW, Blanchet AM, Miossec C, Hervé F, Collard-Dutilleul V, Gu Y, Aldape RA, Lippke JA. A novel human protease similar to the interleukin-1 beta converting enzyme induces apoptosis in transfected cells. EMBO J. 1995;14:1914-1922. [PubMed] |
| 9. | Kumar S, Kinoshita M, Noda M, Copeland NG, Jenkins NA. Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1 beta-converting enzyme. Genes Dev. 1994;8:1613-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 482] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Miossec C, Decoen MC, Durand L, Fassy F, Diu-Hercend A. Use of monoclonal antibodies to study interleukin-1 beta-converting enzyme expression: only precursor forms are detected in interleukin-1 beta-secreting cells. Eur J Immunol. 1996;26:1032-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Gu Y, Wu J, Faucheu C, Lalanne JL, Diu A, Livingston DJ, Su MS. Interleukin-1 beta converting enzyme requires oligomerization for activity of processed forms in vivo. EMBO J. 1995;14:1923-1931. [PubMed] |
| 12. | Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995;182:1625-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 297] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Gansauge S, Gansauge F, Ramadani M, Stobbe H, Rau B, Harada N, Beger HG. Overexpression of cyclin D1 in human pancreatic carcinoma is associated with poor prognosis. Cancer Res. 1997;57:1634-1637. [PubMed] |
| 14. | Toyoda H, Nakamura T, Shinoda M, Suzuki T, Hatooka S, Kobayashi S, Ohashi K, Seto M, Shiku H, Nakamura S. Cyclin D1 expression is useful as a prognostic indicator for advanced esophageal carcinomas, but not for superficial tumors. Dig Dis Sci. 2000;45:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Sallinen SL, Sallinen PK, Kononen JT, Syrjäkoski KM, Nupponen NN, Rantala IS, Helén PT, Helin HJ, Haapasalo HK. Cyclin D1 expression in astrocytomas is associated with cell proliferation activity and patient prognosis. J Pathol. 1999;188:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Keum JS, Kong G, Yang SC, Shin DH, Park SS, Lee JH, Lee JD. Cyclin D1 overexpression is an indicator of poor prognosis in resectable non-small cell lung cancer. Br J Cancer. 1999;81:127-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Korc M, Chandrasekar B, Shah GN. Differential binding and biological activities of epidermal growth factor and transforming growth factor alpha in a human pancreatic cancer cell line. Cancer Res. 1991;51:6243-6249. [PubMed] |
| 18. | Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328-5337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 404] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565-569. [PubMed] |
| 20. | Kornmann M, Ishiwata T, Arber N, Beger HG, Korc M. Increased cyclin D1 expression in chronic pancreatitis. Pancreas. 1998;17:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Korc M, Friess H, Yamanaka Y, Kobrin MS, Buchler M, Beger HG. Chronic pancreatitis is associated with increased concentrations of epidermal growth factor receptor, transforming growth factor alpha, and phospholipase C gamma. Gut. 1994;35:1468-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Gansauge S, Gansauge F, Yang Y, Müller J, Seufferlein T, Ramadani M, Beger HG. Interleukin 1beta-converting enzyme (caspase-1) is overexpressed in adenocarcinoma of the pancreas. Cancer Res. 1998;58:2703-2706. [PubMed] |
| 23. | Ramadani M, Yang Y, Gansauge F, Gansauge S, Beger HG. Overexpression of caspase-1 (interleukin-1beta converting enzyme) in chronic pancreatitis and its participation in apoptosis and proliferation. Pancreas. 2001;22:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |