Published online Dec 15, 2003. doi: 10.3748/wjg.v9.i12.2791
Revised: August 17, 2003
Accepted: September 17, 2003
Published online: December 15, 2003
AIM: Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by visceral hypersensitivity and altered bowel motility. There is increasing evidence suggesting the role of inflammation in the pathogenesis of IBS, which addresses the possibility that formerly established rat model of colitis could be used as an IBS model after the inflammation subsided.
METHODS: Colitis was induced by intracolonic instillation of 4% acetic acid in male Sprague-Dawley rats. The extent of inflammation was assessed by histological examination and myeloperoxidase (MPO) activity assay. After subsidence of colitis, the rats were subjected to rectal distension and restraint stress, then the abdominal withdrawal reflex and the number of stress-induced fecal output were measured, respectively.
RESULTS: At 2 days post-induction of colitis, the colon showed characteristic inflammatory changes in histology and 8-fold increase in MPO activity. At 7 days post-induction of colitis, the histological features and MPO activity returned to normal. The rats at 7 days post-induction of colitis showed hypersensitive response to rectal distension without an accompaning change in rectal compliance, and defecated more stools than control animals when under stress.
CONCLUSION: These results concur largely with the characteristic features of IBS, visceral hypersensitivity and altered defecation pattern in the absence of detectable disease, suggesting that this animal model is a methodologically convenient and useful model for studying a subset of IBS.
- Citation: La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol 2003; 9(12): 2791-2795
- URL: https://www.wjgnet.com/1007-9327/full/v9/i12/2791.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i12.2791
Irritable bowel syndrome (IBS) is defined as a group of functional bowel disorders in which abdominal discomfort or pain is associated with defecation or a change in bowel habit in the absence of an identifiable disease process[1]. Clinical observations have revealed that patients with IBS show a disturbed colonic motor function and hypersensitivity to luminal distension, and these symptoms are generally accepted as cardinal features of IBS[2].
Although IBS is known as one of the most common disorder encountered in clinical practice[3], progress in the study of IBS in the basic scientific research fields has been hindered largely due to the lack of useful animal models that mimic the features of IBS[4]. Since the pathophysiological processes involved in IBS are multifactorial, researchers have employed various kinds of symptom-generating stimulus to establish animal models of IBS. These include a stressful event in adult animals[5], a chemical or mechanical irritation of colon in early life[4], and neonatal maternal separation[6].
Recently, attention has been directed to the role of inflammation in the pathogenesis of IBS[3,7], and IBS after enteric infection, namely post-infectious IBS has begun to be studied using rodent models experimentally infected with Nippostrongylus brasilensis[8] or Trichinella spiralis[9]. The infected animals underwent jejunitis and showed altered intestinal motor function or hypersensitivity to luminal distension even after the pathogens were eliminated. These inspiring reports addressed the possibility that the well-characterized rodent models of colitis could also exhibit some features of post-infectious IBS after resolution of colitis or, at least, in a certain period of time during the recovery course.
In the present study, we examined this possibility using one of the extensively studied rodent models of acute colitis, the rat model of acetic acid-induced colitis. Specifically, we aimed to find out whether the animal could show visceral hypersensitivity to rectal distension and altered defecation pattern under stress after the subsidence of colitis.
Male Sprague-Dawley rats (270-310 g) were housed individually in an access-restricted room with controlled temperature (23 °C) and light-dark cycles (12:12 h). All the experimental protocols in this study were reviewed and approved by the Animal Care and Use Committee of Seoul National University.
After an overnight fast, the rats were lightly anesthetized with ether, and colitis was induced by intracolonic instillation of 1 ml 4% acetic acid (Fluka, Buchs SG, Switzerland) at 8 cm proximal to the anus for 30 s. Then, 1 ml phosphate buffered saline was instilled to dilute the acetic acid and flush the colon. Control animals were handled identically except that 1 ml saline was instilled instead of 4% acetic acid.
MPO activity assay was performed to quantify the inflammation in distal colon according to the procedure described previously[10]. Sixteen rats (4 animals at 2 and 7 days post-enema in each group) were used for this study. These two time-points were selected according to previous reports[10,11] to represent the overt inflammatory phase and the subsiding phase, respectively. Briefly, an 8 cm segment of distal colon was collected via laparotomy, minced in 1 ml of 50 mM potassium phosphate buffer (pH 6.0) containing 14 mM hexadecyltrimethylammonium bromide (Fluka), homogenized and sonicated. The lysates were frozen and thawed three-times, then centrifuged for 2 min in cold at 15000 g. Aliquots of the supernatants were mixed with potassium phosphate buffer containing o-dianisidine-HCl (Sigma-Aldrich, St. Louis, MO, USA) and 0.0005% H2O2. The change in absorbance at 460 nm was spectrophotometrically measured. MPO activity was expressed as units/g of wet tissue. The enzyme unit was defined as the conversion of 1 µmol of H2O2 per min at 25 °C.
To examine the extent of colonic inflammation, histological samples were collected at the selected time points. Sections with a thickness of 5 μm were cut and processed for hematoxylin-eosin staining. The coded slides were analyzed by a pathologist blinded with regard to the treatment group and the time points.
At 7 days post-enema, eight rats from each group were used for studying visceral sensitivity to rectal distension. A disposable silicon balloon-urethral catheter for pediatric use (6 Fr, Sewoon Medical Co., Seoul, Korea) was used for this purpose. The maximal inflation volume for the balloon was 1.0 ml and the length of the maximally inflated balloon was 1.2 cm. After an overnight fast, the animals were lightly anesthetized with ether, and the balloon was carefully inserted into the rectum until the pre-marked line on the catheter (2 cm distal from the end of the balloon) was positioned to the anus, then the catheter was taped to the base of the tail to prevent displacement. After this procedure, the rats were placed in a transparent cubicle (20 cm × 8 cm × 8 cm) on a mirror-based elevated platform while still sedated, and were allowed to recover and acclimate for a minimum of 30 min before testing. The catheter was connected to a pressure transducer (RP-1500, Narco Bio-systems Inc., USA) via a 3-way connector, and a non-invasive pulse transducer (MLT125R, AD Instruments, Castle Hill, Australia) was attached so that the active site of the transducer was located on the ventral surface of the tail, directly below the caudal artery. The signals from both transducers were processed through PowerLab/400 (AD Instruments) and recorded on an IBM-compatible computer.
After the animals were fully awaken and acclimatized, ascending-limit phasic distension (0.1, 0.2, 0.3, 0.4, 0.6, 0.8 and 1 ml) was applied for 30 s every 4 min. The balloon was distended with pre-warmed (37 °C) water. We chose this protocol as hypersensitivity was reported to be best elicited by rapid phasic distension[2]. In this experiment, the abdominal withdrawal reflex (AWR) was semiquantitatively scored as previously described[4] and the concomitant change in arterial pulse rate was measured. The AWR score was assigned as follows: 0 = no behavioral response to distension, 1 = brief head movements followed by immobility, 2 = contraction of abdominal muscle without lifting of abdomen, 3 = lifting of abdomen, 4 = body arching and lifting of pelvic structure.
After the experiments, the balloon was withdrawn and immersed in 37 °C tap water. Since the compliance of balloon was not infinite, we measured intraballoon pressure at each distension volume in 37 °C water, and digitally subtracted the value from that recorded during the rectal distension experiment to calculate the intrarectal pressure.
Fifteen animals of each group were used for this experiment. The rats were housed individually with no restrictions on food intake before testing. At 7 days post-enema, ten rats of each group were placed in restraint cages (5 cm × 5 cm × 20 cm) for 1 hr at room temperature. The feces excreted during restraint stress were divided into three types: hard pellet, soft pellet and formless stool, and counted separately. In another experiment, five rats of each group were left to be unrestrained for 1 hr and served as unstressed control. All the experiments were performed between 1000 and 1200 h.
Data were expressed as mean ± SD. Significant difference between the two groups in MPO activity at the selected time-points and in the values (AWR score and the pulse rate change) at each distension volume was statistically analyzed using Mann-Whitney U-test with P value set at < 0.05 significance level. The relationship between AWR score and the extent of pulse rate change was determined by linear regression analysis, and the estimated slope coefficients and intercepts were compared between groups using Student’s t-test at 2N-4 (N = the number of data points) degrees of freedom (d.f.). The intraballoon volume-intrarectal pressure relationship of each group was also analyzed as above. The number of fecal output was compared using ANOVA and further analyzed using Newman-Keuls multiple comparison test, and considered significantly different from others when P < 0.05.
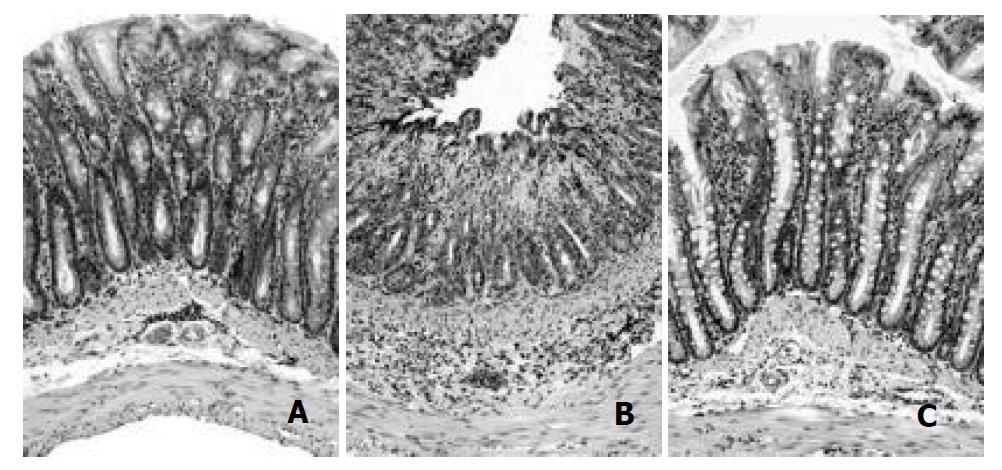
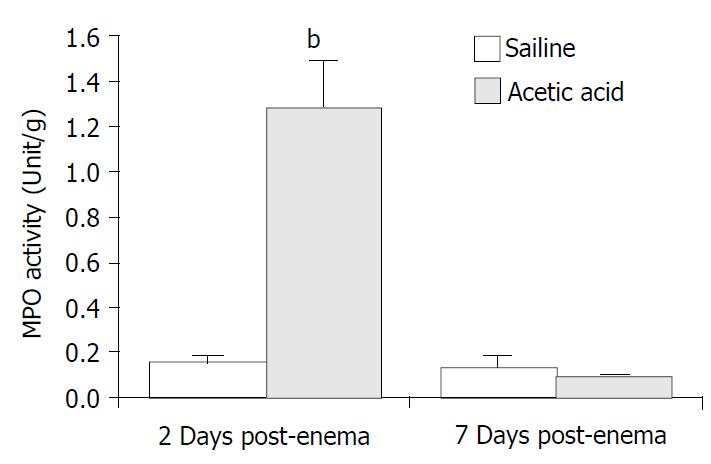
Figure 1 represents the histology of the distal colon in control (A), at 2 days (B) and at 7 days (C) post-induction of colitis (PIC). At 2 days PIC, mucosal hemorrhage with an inflammatory infiltrate in lamina propria and the edematous submucosa was observed. On the other hand, there was no remarkable inflammatory feature at 7 days PIC. Similar result was obtained from the MPO activity assay of colonic tissue. As shown in Figure 2, the enzyme activity was dramatically increased (8-fold) at 2 days PIC (P = 0.0015, n = 4), however it was not significantly different from the control value at 7 days PIC (P > 0.5, n = 4). These results indicated that at 7 days PIC, acute colonic inflammation was in subsiding phase.
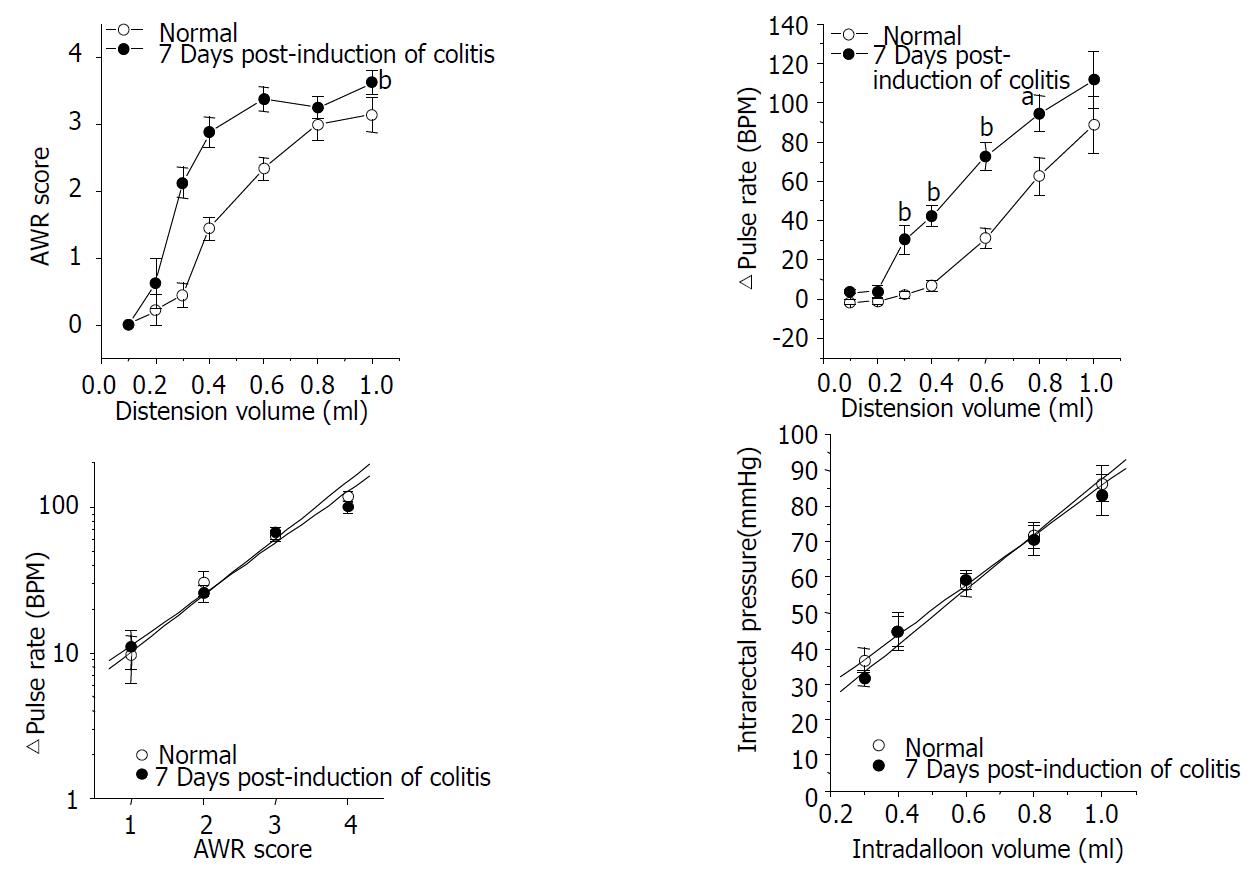
Animals awaken in the cubicle showed regular arterial pulse after about 30 min acclimation. The pulse rate was 345.6 ± 6.2 beat per minute (BPM) in control group (n = 8) and 329.7 ± 7.3 BPM in the 7 days PIC group (n = 8), and the resting pulse rates of the two groups were not significantly different from each other. Pulse rate was instantly increased as the balloon inflated in the rectum and returned to nearly the resting level as it deflated. Animals at 7 days PIC showed hypersensitive response to the ascending-limit phasic rectal distension. The nociceptive threshold (distension volume that produced the AWR of score 2, i.e., abdominal contraction) was about 0.6 ml in the control group, and it was lowered to around 0.3 ml in the 7 days PIC group. The AWR score in rats at 7 days PIC was generally higher than that in control animals, which shifted the distension volume-response curve to left (Figure 3A). In addition, the extent of rectal distension-induced tachycardia was also significantly increased in the 7 days PIC group (Figure 3B).
We further analyzed these results by determining the relationship between the AWR score and the extent of pulse rate change (D pulse rate). The mean value with its standard error of D pulse rate at a given AWR score was plotted in logarithmic scale for a linear fit. As shown in Figure 3C, there was a good linear correlation between the AWR score and the logarithmic D pulse rate (r = 0.99, P < 0.008 in control; r = 0.99, P < 0.007 in the 7 days PIC group). The fitted functions of two groups were not significantly different from each other (slope coefficient: 0.39 ± 0.03 in control vs. 0.35 ± 0.01 in the 7 days PIC group (P > 0.45, d.f. = 4), intercept: 0.62 ± 0.07 in control vs. 0.69 ± 0.06 in the 7 days PIC group (P > 0.43, d.f. = 4)). This implied that scoring the AWR in the present study was a reliable method to quantify the animal’s nociceptive response to rectal distension.
In order to examine whether the visceral hypersensitivity in rats at 7 days PIC was related to changes in rectal compliance, we compared the intraballoon volume-intrarectal pressure relationship of the two groups. The distension volume from 0.3 ml to 1.0 ml and the corresponding value of calculated intrarectal pressure (refer MATERIALS AND METHODS) were plotted for regression analysis (Figure 3D). Intrarectal pressure was linearly increased as the balloon inflated (r > 0.99, P < 0.0001 in control; r = 0.99, P = 0.0015 in the 7 days PIC group). The fitted functions of the two groups were not significantly different from each other (slope coefficient: 69. 83 ± 0.99 in control vs. 77.46 ± 6.81 in the 7 days PIC group (P > 0. 3, d.f. = 6), intercept: 15.94 ± 0.64 in control vs. 10.15 ± 3.67 in the 7 days PIC group (P > 0.17, d.f. = 6)).
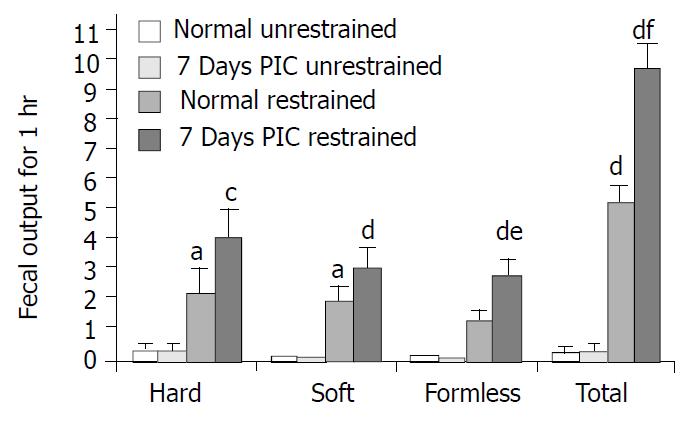
As shown in Figure 4, the number of fecal pellet output for 1 hr was negligible in unrestrained animals regardless of the treatment. However, restraint stress significantly increased defecation. The restrained control rats defecated 2.1 ± 0.9 hard pellets, 1.9 ± 0.4 soft pellets, 1.2 ± 0.4 formless stool and a total of 5.2 ± 0.5 feces in 1 hr (n = 10). Comparatively, animals at 7 days PIC group defected 4 ± 0.9 hard pellets, 3 ± 0.6 soft pellets, 2.7 ± 0.6 formless stool and 9.7 ± 0.8 feces in total during the 1 hr restraint stress (n = 10). The number of formless stool and the total number of fecal output during 1 hr restraint stress in the 7 days PIC group were significantly larger than the corresponding values in control rats, which implied that the stress-induced increase in colonic motor function was enhanced in rats at 7 days PIC group.
In human study, patients with ulcerative colitis were reported to show visceral hypersensitivity even when their disease was in quiescent state[12]. In the present study, we found that 7 days after the acetic acid-induced colitis subsided, the rats still showed visceral hypersensitivity, a generally accepted common feature of patients with IBS. Moreover, the rats at 7 days PIC defecated more stools in response to restraint stress than control animals, which was in good accordance with the clinical observations reporting the higher incidence of urgency to defecate and altered stool pattern under stress in IBS patients[13]. These results suggested that along with the previously reported parasite-infected rat models, acetic acid-induced colitis model could be used for the study of a subset of IBS that developed from intestinal inflammation (post-inflammatory IBS).
Indeed, it was well documented that colitis caused motility alteration and visceral hypersensitivity in various models. For instance, trinitronbenzene sulfonic acid (TNBS)/ethanol-induced colitis lowered the threshold for colorectal distension-induced visceromotor reflex at 3 days post-enema[14], and progressively altered colonic muscle function[15]. Similarly, acetic acid-induced colitis increased visceromotor response to colorectal distension at 6 and 24 h post-enema[16], and decreased colonic circular muscle contractility[17]. However, these studies mainly dealt with the alterations in acute or overt inflammatory phase, and few studies focused on the response of the animals with ‘once’ inflamed colon.
Advantages of our model as a post-inflammatory IBS model include not only a relatively short period required for the subsidence of inflammation but also an easy access to the affected sites, i.e., rectum and colon, which make it possible to measure behavioral and pseudoaffective responses in the awake animals. In the nematode-infected IBS models, the affected site is jejunum to which anesthesia and surgical procedures are required to access for experiments. Another potential advantage is a large body of literatures reporting the pathophysiological mechanisms of alterations occurred during active colitis. Scrutinizing those reports might help to find the pivotal inflammatory changes that induce the IBS symptoms.
The reasons for the exaggerated responses to rectal distension and restraint stress in our model have been unclear, but at least, it seemed not related to changes in rectal compliance since the intraballoon volume-intrarectal pressure relationship was not different between the groups. It was noteworthy that mast cells were found to be increased in a group of IBS patients[3], and partially mediate functional disorder in the N. brasiliensis-infected rat model of post-infectious IBS[8]. We are currently investigating whether mast cell also plays a role in the post-colitis IBS features in rat.
In summary, we found that the rat model of acetic acid-induced colitis showed major features of IBS after subsidence of the acute inflammation in colon. The results suggest that this model has several advantages as a useful model of post-inflammatory IBS.
This work was supported by the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University.
Edited by Zhu LH
| 1. | Camilleri M, Heading RC, Thompson WG. Clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:1407-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51 Suppl 1:i67-i71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome. Gut. 2002;51 Suppl 1:i41-i44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 191] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 554] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 5. | Bradesi S, Eutamene H, Garcia-Villar R, Fioramonti J, Buéno L. Acute and chronic stress differently affect visceral sensitivity to rectal distension in female rats. Neurogastroenterol Motil. 2002;14:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307-G316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 8. | Gay J, Fioramonti J, Garcia-Villar R, Buéno L. Alterations of intestinal motor responses to various stimuli after Nippostrongylus brasiliensis infection in rats: role of mast cells. Neurogastroenterol Motil. 2000;12:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997;113:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Al-Awadi FM, Khan I. Studies on purine enzymes in experimental colitis. Mol Cell Biochem. 1999;194:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Conner EM, Grisham MB. Animal models of colitis. In: Gaginella TS, eds. Experimental models of mucosal inflammation. New York: CRC press. 1996;97-109. |
| 12. | Rao SS, Read NW, Davison PA, Bannister JJ, Holdsworth CD. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology. 1987;93:1270-1275. [PubMed] |
| 13. | Drossman DA, Sandler RS, McKee DC, Lovitz AJ. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology. 1982;83:529-534. [PubMed] |
| 14. | Morteau O, Hachet T, Caussette M, Bueno L. Experimental colitis alters visceromotor response to colorectal distension in awake rats. Dig Dis Sci. 1994;39:1239-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Hosseini JM, Goldhill JM, Bossone C, Piñeiro-Carrero V, Shea-Donohue T. Progressive alterations in circular smooth muscle contractility in TNBS-induced colitis in rats. Neurogastroenterol Motil. 1999;11:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Burton MB, Gebhart GF. Effects of intracolonic acetic acid on responses to colorectal distension in the rat. Brain Res. 1995;672:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Myers BS, Martin JS, Dempsey DT, Parkman HP, Thomas RM, Ryan JP. Acute experimental colitis decreases colonic circular smooth muscle contractility in rats. Am J Physiol. 1997;273:G928-G936. [PubMed] |