INTRODUCTION
Gastrointestinal hormones such as gastrin and somatostatin, regulate the secretion, motility, absorption, blood flow and cell nutrition of the digestive tract. Abnormality of their secretion often affects the normal functions of digestive tract, even cause clinical symptoms or syndromes. Pathological impairment of gastrointestinal tract could also result in changes of the level of gastrointestinal hormones. Gastrin is mainly secreted from gastrin secreting cells (G cells) in antrum mucosa or upper small intestine. Medulla oblongata and dorsal nuclei of vagus nerve in central nervous system also have gastrin. Somatostatin is distributed in the body, hypothalamus and at other sites of the brain, peripheral nerve and gastrointestinal tract. In digestive system, for example, somatostatin is secreted from somatostatin secreting cells (D cells). D cells are distributed mainly in intestinal nerve plexus, stomach and pancreas[1-4].
Although there are some methods to observe the shape of G cells and D cells, microscope or electron microscope could not decide G cells or D cells alone. Immunohistochemical method could not demonstrate G cells or D cells at ultrastructural levels. Thus investigations at the ultrastructural level by immunoelectron microscopy are effective[5-8]. This study was to demonstrate G cells and D cells at the ultrastructural level by colloidal gold labeled immunoelectron microscopy technique.
MATERIALS AND METHODS
Guinea pigs and antral tissue processing
Seven healthy male Wistar rats weighing 230-250 g from the Center of Experimental Animals in Sun Yat-sen University of Medical Sciences (Guangzhou, China) were used. All rats received no special treatment before sacrificed. The rats were fasted overnight with free access to water. Four days later, the rat’s abdomen, anesthetized with 3% of sodium pentobarbital intraperitoneally at a dosage of 30 mg/kg, was cut open and its stomach was split from the greater curvature. The antral tissue of about 0.5 mm×0.5 mm was separated using ophthalmic scissors. Then the specimens were immersed into a mixture of 0.1% glutaraldehyde and 3% paraformaldehyde in 0.1M PBS, pH7.4, for 2 hr at room temperature for fixation.
Specimen preparation for immunoelectron microscopy
Two hours after fixation, the antral tissue specimens were washed four times for 15 min in 0.1M PBS, pH7.4, and then postfixed for 1 hr in solution of 1% osmium tetroxide (1% potassium dichromate, pH 7.2, 1% osmium tetroxide, 0.85% NaC1) at room temperature. The specimens were washed three times for 10 min in 0.1M PBS, pH7.4, and dehydrated at room temperature in 50% acetone (15 min), 70% acetone (15 min), 90% acetone (15 min), and 100% acetone (three times for 15 min each). The specimens were then infused in an open desiccator containing 50% acetone: 50% Spurr resin (1 hr), 33% acetone: 67% Spurr resin (2 hr), and 100% Spurr resin overnight. When the resin was infused in the specimens, it was polymerized at 40 °C for 4 days. To orientate the samples, 1 μm thick sections were cut, put on an objective glass, and stained with 0.1% teluidin blue. Appropriate regions were chosen, and the pyramids were further trimmed, cut on a Leica Rechert ultramicrotome into 60-80 nm ultrathin sections, put onto 300 nickle mesh grids. All ultrathin sections were divided into G cells group, D cells group, and control group.
Postembedded antibody incubation and immunoelectron microscopy
All the ultrathin sections were oxidized in H2O2 for 10 min, washed three times for 5 min in water. Osmium tetroxide was removed in 1% sodium periodate, washed three times for 5 min in 0.05 M TBS, pH7.4. The ultrathin sections were incubated for 30 min at room temperature in 1.5% BSA (Sigma, USA) in PBS for blocking. The sections of G cells group were then incubated with rabbit anti-gastrin polyclonal antibody (Sigma, USA) at a concentration of 1:80 in PBS, the sections of D cells group were incubated with rabbit anti-somatostatin polyclonal antibody (ZYMED, USA) at a concentration of 1:80 in PBS, and the sections of control group were incubated with PBS only. All sections were incubated in a wet box at 4 °C overnight and then at 37 °C for 1 hr. The sections were washed three times for 10 min in 0.05M TBS, pH7.4, then one time for 10 min in 0.02 M TBS, pH7.4. The sections were again incubated for 30 min at room temperature in 1.5% BSA in PBS for blocking and then incubated with a gold-conjugated secondary antibody, colloidal-gold/staphylococcic-protein-A 1:80 (10 nm, Boster Biological Technology Co, Wuhan, China) in PBS, for 1 hr at 37 °C. The sections were washed three times for 5 min in water and stained with 5% uranyl acetate (in water) at room temperature for 60 min, and with lead citrate for 60 min. At last the specimens were examined and photographed under an electron microscope (Philips CM10).
Image analysis of colloidal gold granules
A total of 50 cells in either G cells group or D cells group were randomly photographed from sections of their photos. They were input into the Quantimet-500 image analysis system (Leica Co. German) for calculation of the average number of colloidal gold granules. The data thus obtained were expressed in -x±s.
RESULTS
Immunoelectron microscopy of G cells
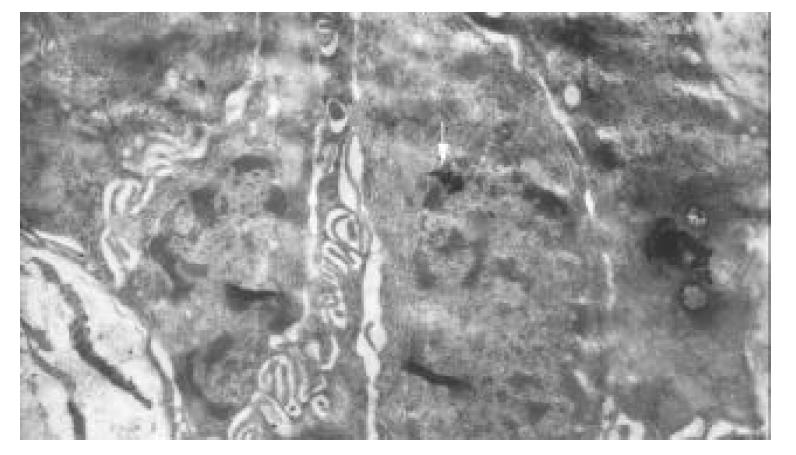
No immunological granules of colloidal gold in most antral mucosa cells were found, except those in G cells. Immunological granules of colloidal gold were well distributed in G cells, with the cells’ ultrastructure clearly fixed. While the membrane of G cells was intact, rough endoplasmic reticules were increased, and mitochondria decreased. The nuclei of G cells were normal, and the chromatin was equally distributed. The gastrin labeled golden granules in G cells were clear, either round-shaped or oval-shaped, with a high electro-density. The granules presented mainly as lobation-like or island-like congeries, which were dispersed in cytoplasms of G cells, directing toward the basement membrane. A few golden congeries in the nuclei of G cells were mainly located in euchromatin area. No golden congeries were observed in heterochromatin area. There were immunological granules of colloidal gold in the nuclei of G cells as island-like congeries and penetrating nuclear pore in cytoplasms (Figure 1). The number of golden granules in a G cell was 107.04 ± 19.68.
Figure 1 Immunological granules of colloidal gold located in nuclei of G cell as island-like congeries (↑), and penetrating nuclear pores into cytoplasms.
×21000.
Immunoelectron microscopy of D cells
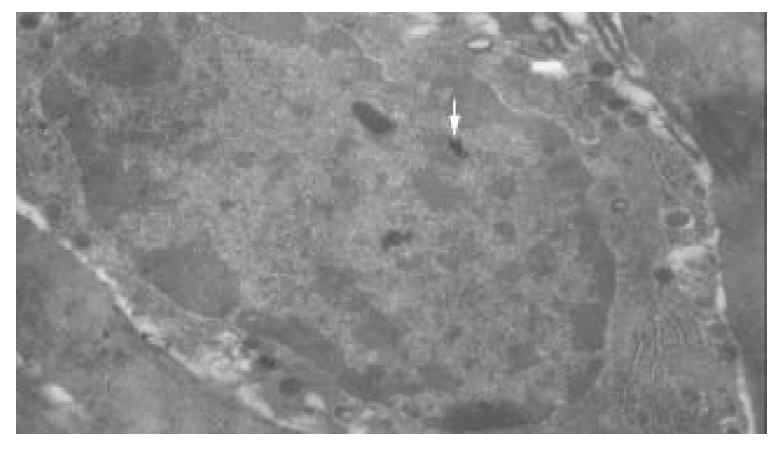
No immunological granules of colloidal gold were found in most antral mucosa cells, except those in D cells. Immunological granules of colloidal gold were found well distributed in D cells, with the ultrastructure clearly fixed. The membrane of D cells was intact, rough endoplasmic reticules were increased, and mitochondria decreased. The nuclei of D cells were normal, with their chromatin well distributed. The somatostatin labeled golden granules in D cells were clear, either round-shaped or olive-shaped, with a high electro-density. The granules presented mainly as lobation-like or island-like congeries, a few of them were dispersed in D cells while most of them were dissociated in cytoplasms of D cells, directing toward the basement membrane. Again, a few golden congeries in the nuclei of D cells were mainly located in euchromatin area (Figure 2). No golden congeries were found in heterochromatin area. The number of golden granules in a D cell was 83.36 ± 17.58. No colloidal gold-labeled cell was observed in control group.
Figure 2 Immunological granules of colloidal gold locate in nuclei of D cell as island-like congeries (↑).
×8900.
DISCUSSION
Gastric mucosa consists of highly organic multi-functional glands. In a single gastric gland there are at least five kinds of cells. They are the parietal cell, chief cell, endocrine cell, superficial epithelial cell and mucous cell. The stem cells of all these kinds of cells are in the neck area near the surface of the gland. When migrating upward to the surface of gland, they changed into the surface mucous cells. When migrating downward deeper into the gland, the proliferative stem cells changed into cells in forms of parietal cells, chief cells, and endocrine cells[9-11].
The features of gastrin can be briefly summarized as follows. There are gastrin receptors both in parietal cells and in ECL cells. As the main regulating hormone of gastric acid secretion, gastrin stimulates parietal cells to secrete gastric acid directly via the gastrin receptors in parietal cells and also stimulates ECL cells to secrete histamine via the gastric receptors in the cells. Then histamine stimulates parietal cells to secrete gastric acid via H2 receptors in parietal cells in a paracrine style. While accelerating the secretion of pepsin, secretin, pancreatic juice, and the secretion of water and salt in bile, gastrin also speeds up the releasing of insulin and calcitonin. It has both nutritional and accelerating effects on gastric mucosa. It also has a growth factor producing the similar effect. In the same way, the increase of gastrin would lead to the increasing number of both parietal cells and ECL cells. Apart from all these, gastrin is also closely related with the occurrence of carcinoid tumor. Gastrin enhances the motion of gastrointestinal tract, thus increasing the contraction effect of the stomach, intestine and gall bladder. In addition, there are reports that G cells should be taken as the fourth histamine source in stomach besides chief cells, ECL cells, and intestinal nerve plexus. Increase of pH, intake of meal, increase of pressure in the stomach, and excitation introduced by vagus nerve or mucosa nerve plexus could make G cells secrete more gastrin. Decrease of pH stimulated by somatostatin or other gastrointestinal hormones, and excitation of sympathetic nerve could inhibit the secretion of gastrin[12-17].
What characterizes somatostatin is presented as follows. The main physiological effect produced by somatostatin on digestive system is inhibition. Somatostatin could inhibit peristalsis of stomach, intestine, gall bladder and proliferation of mucosal cells, reduce blood flow of gastrointestinal tract and small intestine to absorb water, electrolyte, glucose, amino acid, and triglycerides, depress the secretion of gastric acid, pepsin, hepatic bile, small intestinal juice, and secretion of gastrin and other gastrointestinal hormones. Excitation of sympathetic nerve could inhibit D cells to secrete somatostatin, but that of vagus nerve could stimulate D cells to do so. Gastrin has a unique regional regulatory effect on secretion of somatostatin in stomach. Gastrin could directly stimulate D cells to secrete somatostatin. In some species, gastrin receptors have been detected in D cells of stomach. As shown in some investigations, endogenous histamine inhibited the secretion of somatostatin via paracrine effect on H3 receptor. Others showed that endothelins inhibited the secretion of somatostatin via endothelin receptors[18-23].
In histocyte, there are often different granules that are important symbols of classification. This includes neurosecretory granule of 80-600 nm with an electron-densed core, highly electron-densed zymogen granule of 1-2 μm, large and light mucus granules and endocrine secretory granules. Although some endocrine secretory granules in endocrine cells had definite characteristics and are somewhat significant to evaluate the type of such endocrine cells, it was difficult to differentiate the features of endocrine simply according to endocrine cells and their secretory granules[24-28].
Colloidal gold immunoelectron microscopic technique is the advanced method for microcosmic morphology. The colloidal gold probe is of high specification, high resolution, accurate localization and accurate quantity. The significance of colloidal gold immunoelectron microscopy is similar to common immunohistochemistry in testing specific antigens expressed in target cells. But it could not be more useful for immunoelectron microscopy to study the localization or the quantitative analysis of antigens at the ultrastructural level. There are many intervening factors in immunoelectron microscopy, the most important one is to protect the activity of antigen. In this aspect, improvement has been made in manipulating the integrating of samples, the fixative and embedding medium, the time of fixation, the temperature of aggregation, the purity and diluting ratio of antibody and colloidal gold, as well as the time of incubation[29-32].
On the whole, the result was satisfactory. The ultrastructure of G cells and D cells was well protected. The ultrastructures of secretory granules of both gastrin and somatostatin were definitely localized. The secretory granules of gastrin in cytoplasms of G cells were also found in the nuclei of G cells. The secretory granules of somatostatin in cytoplasms of D cells were also found in the nuclei of D cells. Therefore, the conclusion is thus made that there are secretory granules of gastrin or somatostatin in nuclei of G cells or D cells. After they are gradually matured, the secretory granules penetrate the nuclear pores into cytoplasms. At last, gastrin or somatostatin is secreted through the basement membrane into intercellular substances, playing their physiological roles. The lobe-like or island-like congeries of colloidal gold observed in G cells and D cells are in coincidence with the existing patterns of the secretory granules of gastrin or somatostatin. In our opinion, the size of secretory granules in congeries of colloidal gold could be subjected to quantitative analysis, though the quantitative scale of each piece of colloidal gold to bind gastrin or somatostatin has not been exactly decided. In this sense, the specific significance of colloidal gold number in congeries of G cells or D cells remains to be precisely interpreted.