INTRODUCTION
It is reported that oil A appears to exert anti-cancer effect by activating apoptosis. It should be speculated that oil A may be broadly active against pancreatic cancer cells. However, whether the use of oil A can be extended to pancreatic tumors is still uncertain. The alternative mechanisms of oil A effect need to be further studied. Our previous experiments showed the inhibition of proliferation and the induction of apoptotic effect by treatment of oil A in pancreatic cancer cells[1]. However, to date, no further information is available regarding the mechanism of effects of oil A on pancreatic cancer cells. In the present study, the mechanism of oil A effect on induction of apoptosis was investigated through activating caspase cascade, inducing cytochrome c release from the mitochondria, Bax, Bcl-2, and Mcl-1 expression, the distribution of cell cycle, changes of cycle-regulating proteins, P21 and P27 expression, and GADD expression in pancreatic cancer cells.
MATERIALS AND METHODS
Reagents
The human pancreatic cancer cell lines, MiaPaCa-2 and AsPC-1 were purchased from the American Type Culture Collection (Rockville, MD, USA). Oil A (Coastside Research Chemical Co., USA) was dissolved in 1:2 DMEM as a stock solution. The stock solution was diluted to appropriate concentrations in serum-free medium prior to experiments. Mitochondria/ Cytosol Fractionation Kit was purchased from BioVision (Mountain View, CA, USA). Enhanced chemiluminescence system (ECL) was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Mouse monoclonal antibodies against PARP, Bcl-2, Bax, Mcl-1, cyclin B1, cyclin D1, cyclin E, CDK2, CDK4, CDK6, P21, P27, GADD45, GADD153 and rabbit polyclonal antibodies against caspase-3, caspase-7, caspase-9, cytochrome c and cyclin A were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All other chemicals were purchased from Sigma (St Louis, MO, USA).
Cell culture
Cells were cultured in DMEM medium supplemented with penicillin G (100 U/mL), streptomycin (100 U/mL) and 10% FBS at 37 °C in humidified air with 5% CO2. The cells were harvested by incubation in trypsin-EDTA solution for 10-15 minutes. Then cells were centrifuged at 300×g for 5 minutes and cell pellets suspended in fresh culture medium prior to seeding into culture flasks or plates[1].
Analysis of cellular DNA content by flow cytometry
The cells were grown to 50%-60% confluence in T75 flasks, serum-starved for 24 hours and then treated with or without 1:32000 oil A for 24 hours. At the end of treatment, the cells were harvested with trypsin-EDTA solution to produce a single cell suspension. The cells were then pelleted by centrifugation and washed twice with PBS. Cell pellets were then suspended in 0.5 ml PBS and fixed in 5 mL ice-cold 70% ethanol at 4 °C. The fixed cells were centrifuged at 300×g for 10 minutes and the pellets were washed with PBS. After resuspension with 1 ml PBS, the cells were incubated with 10 μL of RNase I (10 g/L) and 100 μL of propidium iodide (400 mg/L; Sigma) and shaken for 1 hour at 37 °C in the dark. Samples were analyzed by flow cytometry. The red fluorescence of the single events was recorded using a laser beam at 488 nm excitation λ with 610 nm as emission λ to measure the DNA index.
Preparation of cytosolic and mitochondrial extract
Collect cells (5 × 107) were centrifuged at 600×g for 5 minutes at 4 °C from control and oil A-treated MiaPaCa-2 and AsPC-1 cells. Wash cells with ice-cold PBS twice, and centrifuge at 600×g for 5 minutes at 4 °C. Remove supernatant. Resuspend cells with 1.0 mL of 1×cytosol extraction buffer mix containing DTT and protease inhibitors. Incubate on ice for 10 minutes. Homogenize cells in an ice-cold dounce tissue grinder (45 strokes) until 70%-80% of the nuclei did not have the shiny ring. Transfer homogenate to a 1.5 mL microcentrifuge tube, and centrifuge at 700×g for 10 minutes at 4 °C. Transfer the supernatant to a fresh 1.5 mL tube, and centrifuge at 10000×g for 30 minutes at 4 °C. Collect the supernanant as cytosolin fraction. Resuspend the pellet in 0.1 mL mitochondrial extraction buffer mix containing DTT and protease inhibitors, vertex for 10 seconds and save as mitochondrial fraction. Both cytosolic fraction and mitochondrial fraction were stored at -80 °C until ready for Western blotting.
Preparation of proteins
Cells were seeded into flasks and grown to 50%-60% confluence, then seeded in serum-free medium for 24 hours. Cells were then placed in serum-free medium with or without 1:32000 oil A for a period of 24 hours. In the end, proteins were extracted from attached and floating cells in lysis buffer [20 mM Tris-HCl (pH 7.4), 2 mM sodium vanadate, 1.0 mM sodium fluoride, 100 mM NaCl, 2.0 mM phosphate substrate, 1% NP40, 0.5% sodium deoxycholate, 20 mg/L each aprotinin and leupeptin, 25 mg/L pepstatin, and 2.0 mM each EDTA and EGTA] for further analysis. Protein concentrations were determined using the bicinchoninic acid assay with BSA as standard.
Western blotting
Western blotting was carried out using standard techniques. In brief, equal amounts of proteins in the cell lysates were separated on SDS-PAGE and the proteins then transferred onto nitrocellulose membranes. After blocking non-specific sites with fat free dried milk, membranes were incubated with the appropriate dilution of primary antibody. Then, membranes were incubated with a horseradish peroxidase conjugated secondary antibody. Proteins were detected using an enhanced chemiluminescence detection system, and light emission was captured on Kodak X-ray films.
RESULTS
Effect of oil A on activation of caspase 3 and cleavage of PARP, caspase 7 and caspase 9
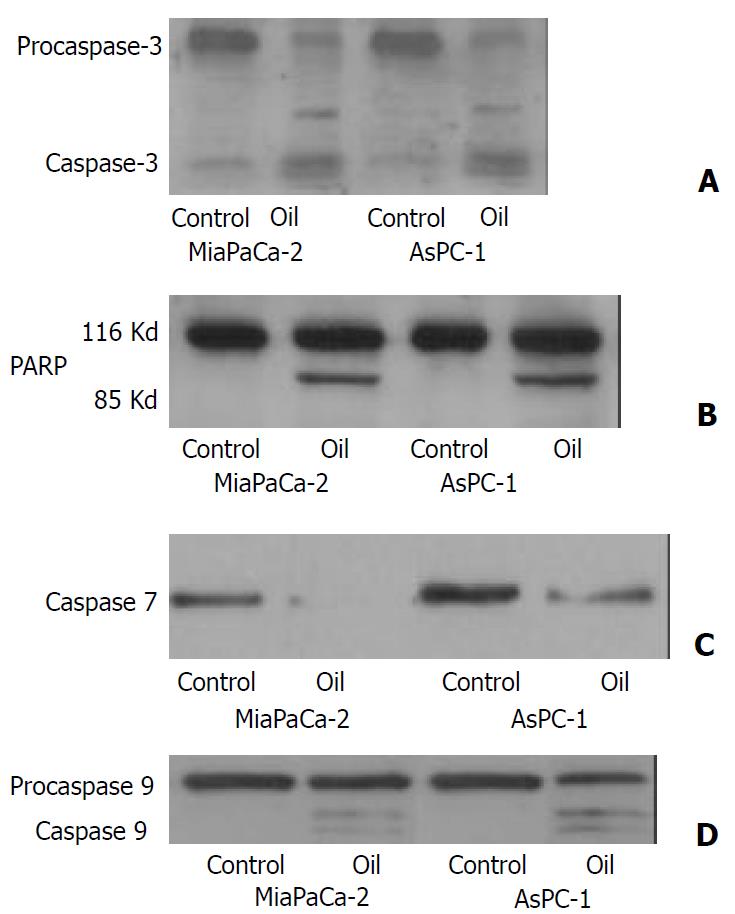
The expression and activation of caspase 3 by cleavage as well as the specific cleavage of its downstream substrate, PARP during apoptosis were observed by Western blotting. The 32 kDa procaspase 3 was cleaved into products of lower molecular weight, including a band corresponding to the 17 kDa active form. The uncleaved 116 kDa pro-form of PARP was only seen in untreated controls while in oil A treatment resulted in appearance of the active 85 kDa cleaved fragments. Caspase 7 and 9 degradation activation were also induced, and coincident with the induction of apoptosis (Figure 1).
Figure 1 (A, B, C, D) Western blotting of the caspase-3, poly-ADP ribose polymerase (PARP), caspase 7 and caspase 9 in MiaPaCa-2 and AsPC-1 cells treated with 1:32000 oil A for 24 hours.
Proteins in whole cellular lysates were electrophoresed in SDS-PAGE gels and transferred to nitrocellulose membranes. Caspase-3, PARP, caspase 7 and caspase 9 were identified us-ing specific antibodies.
Oil A induces cytochrome c release
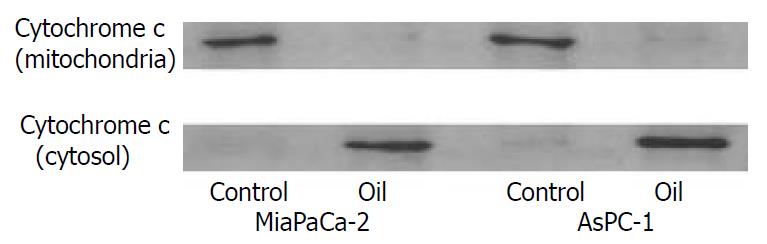
Cytochrome c is a mitochondrial protein released from the mitochondria to cytosol during apoptosis when mitochondrial membrane permeability is disrupted. An increase in the amount of cytochrome c in the cytosolic fraction was seen in both cell lines after oil A treatment. Meanwhile, the amount of cytochrome c in the mitochondrial fraction was decreased also in both cell lines after oil A treatment (Figure 2).
Figure 2 Western blotting of the cytochrome c protein in MiaPaCa-2 and AsPC-1 cells treated with 1:32000 oil A for 24 hours.
Proteins in cytosolic fraction and in mitochondria frac-tion were extracted. Proteins extracted from each sample were electrophoresed on an SDS-PAGE gel and transferred to nitro-cellulose membrane. Cytochrome c was identified using a monoclonal cytochrome c antibody.
Effect of oil A on expression of Bax, Bcl-2, and Mcl-1 proteins
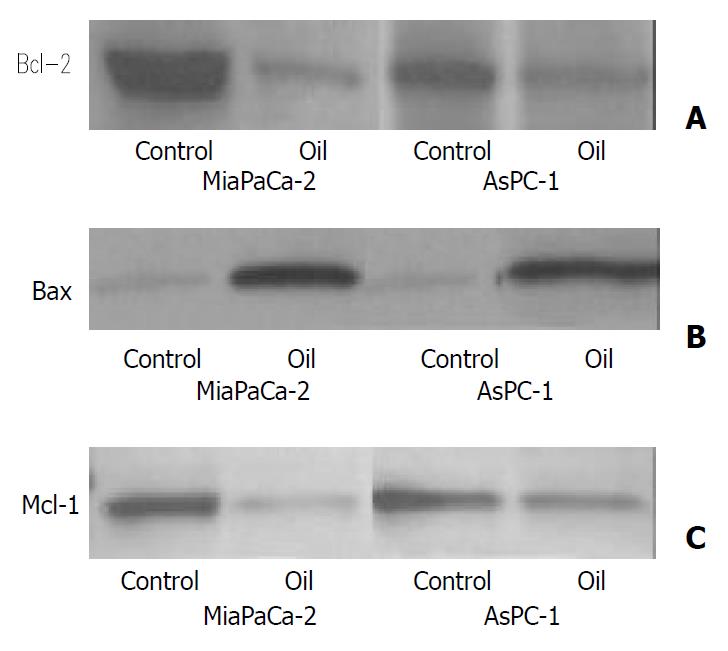
Expression of the anti-apoptosis proteins Bcl-2 and Mcl-1 was decreased and Bax increased following oil A treatment (Figure 3)
Figure 3 (A, B, C) Western blotting of the Bcl-2, Bax and Mcl-1 proteins in MiaPaCa-2 and AsPC-1 cells treated with 1:32000 oil A for 24 hours.
Whole cellular lysates were electrophore-sed in SDS-PAGE gels and proteins were transferred to nitro-cellulose membranes. Bcl-2, Bax and Mcl-1 were identified us-ing specific antibodies.
Effect of oil A on cell cycle phase distribution
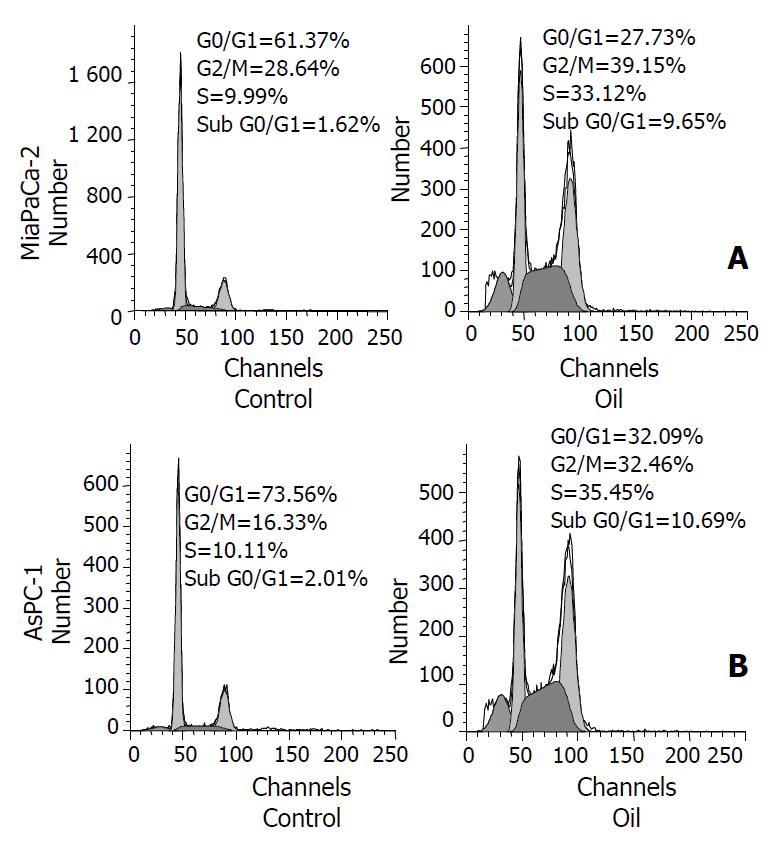
To understand the mechanism of induction of cell apoptosis, the redistribution of cell cycle phases were analyzed after the treatment with 1:32000 oil A for 24 hours. The proportion of cells in the G0/G1 phase decreased in MiaPaCa-2 and AsPC-1 cell lines. Therefore, cells were significantly accumulated in the G2/M-phase in these two cancer cell lines. The number of cells in S-phase was increased also in both cell lines when compared with control. The cells with a sub-G0/G1 DNA content, a hallmark of apoptosis, were seen following 24-hour exposure to oil A in both cell lines (Figure 4).
Figure 4 (A, B) Flow-cytometric analysis of cellular DNA con-tent in control and oil A-treated MiaPaCa-2 and AsPC-1 cells, stained with propidium iodide.
The cells were treated with 1:32000 oil A in serum-free conditions for 24 hours. The dis-tribution of cell cycle phases is expressed as% of total cells.
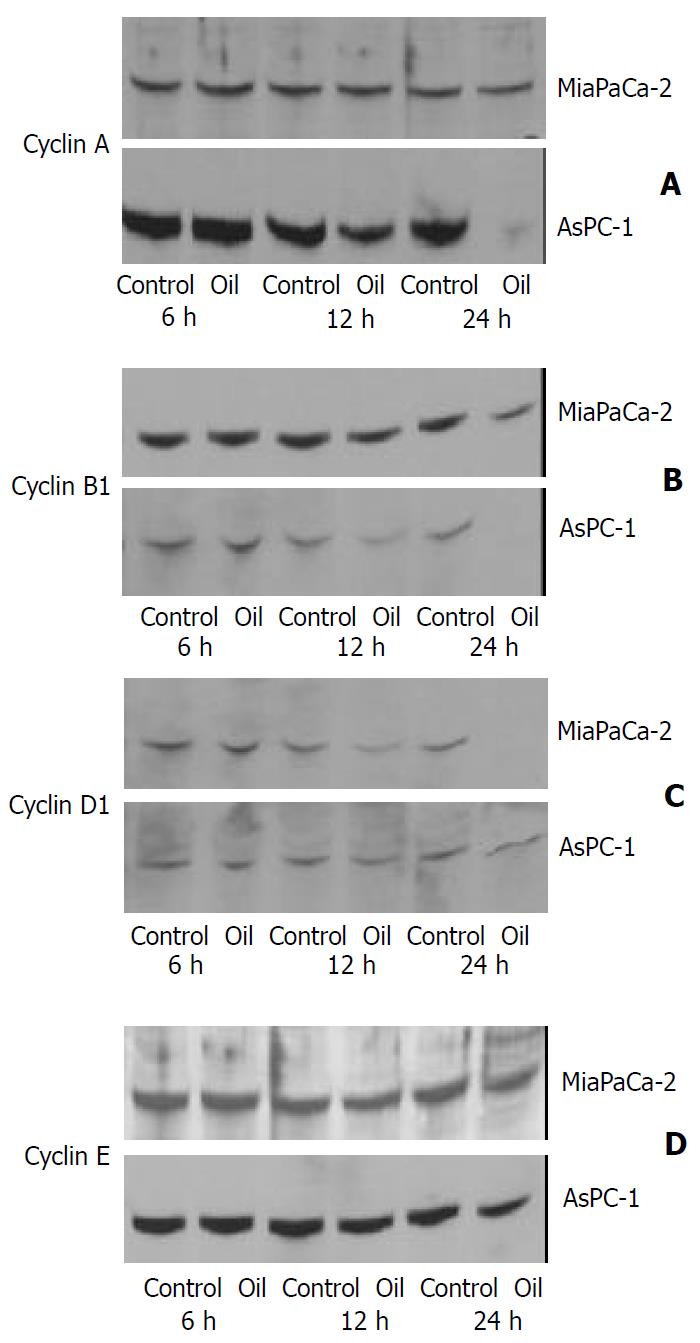
Effect of oil A on expression of cyclin proteins
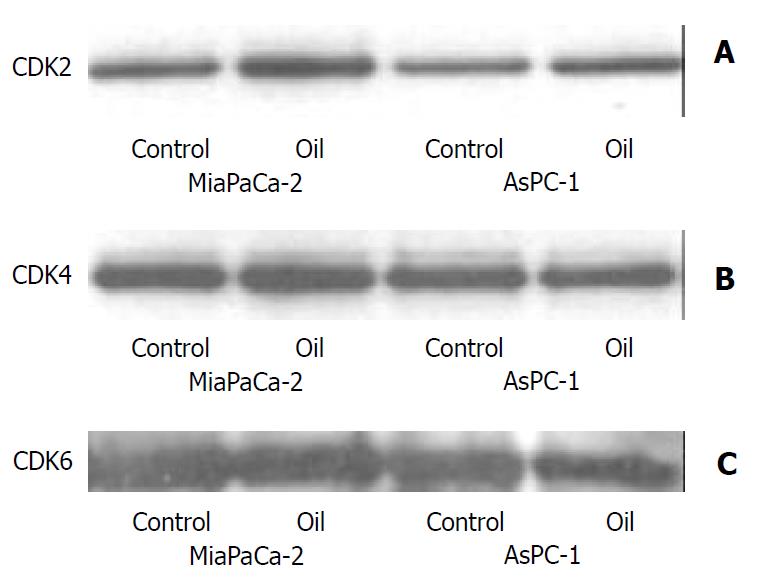
The expression of cyclin A and cyclin B1was slightly decreased and cyclin D1 levels were markedly decreased in MiaPaCa-2 cell line. The expression of cyclin A and cyclin B1 was markedly decreased and cyclin D1 levels were slightly decreased in AsPC-1 cell line, while cyclin E was unaffected and the levels of CDK2, CDK4, and CDK6 remained unchanged in MiaPaCa-2 and AsPC-1 cell lines (Figure 5 and Figure 6).
Figure 5 (A, B, C, D) Western blotting of the cyclin A, cyclin B1, cyclin D1 and cyclin E proteins in MiaPaCa-2 and AsPC-1 cells treated with 1:32000 oil A for 24 hours.
Whole cellular lysates were electrophoresed in SDS-PAGE gels and proteins were transferred to nitrocellulose membranes.
Figure 6 (A, B, C) Western blotting of the CDK2, CDK4 and CDK6 proteins in MiaPaCa-2 and AsPC-1 cells treated with 1:32000 oil A for 24 hours.
Whole cellular lysates were elec-trophoresed in SDS-PAGE gels and proteins were transferred to nitrocellulose membranes.
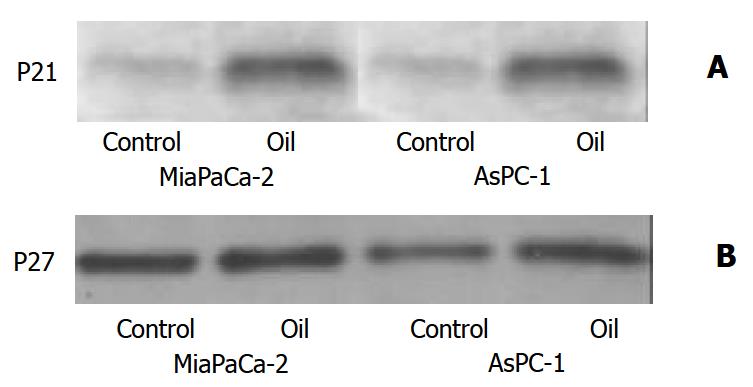
Effect of oil A on expression of P21 and P27 proteins
In response to red oil A, P21 expression was increased, but P27 expression was not affected (Figure 7).
Figure 7 (A, B) Western blotting of the P21 and P27 proteins in MiaPaCa-2 and AsPC-1 cells treated with 1:32000 oil A for 24 hours.
Whole cellular lysates were electrophoresed in SDS-PAGE gels and proteins were transferred to nitrocellulose membranes. P21 and P27 were identified using specific antibodies.
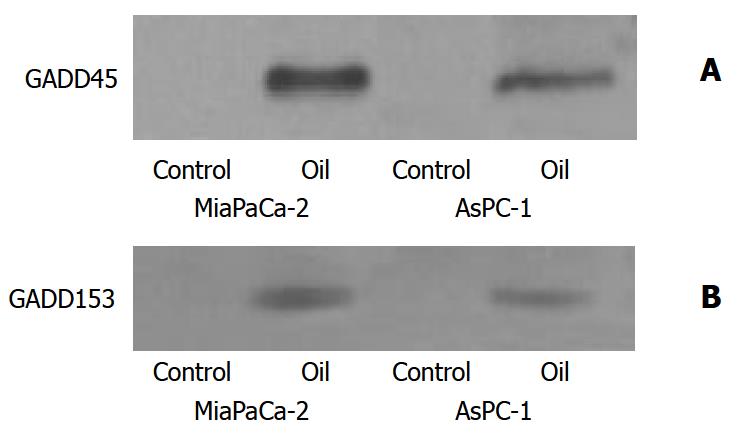
Effect of oil A on expression of GADD
The expression of GADD 45 and GADD153 was enhanced in both pancreatic cancer cell lines following oil A treatment (Figure 8).
Figure 8 (A, B) Western blotting of GADD45 and GADD153 proteins in MiaPaCa-2 and AsPC-1 cells treated with 1:32000 oil A for 24 hours.
Whole cellular lysates were electrophore-sed in SDS-PAGE gels and proteins were transferred to nitro-cellulose membranes. GADD was identified using specific monoclonal antibodies.
DISCUSSION
Because of its significant medical properties, fish oil has been used for many years.
Recent studies indicate that diets containing a high proportion of long-chain n-3 polyunsaturated fatty acids were associated with inhibition of the growth and metastasis of human cancer including pancreatic cancer[1-3]. Diets rich in linoleic acid (LA), an n-6 fatty acid, stimulate the progression of human cancer cell in athymic nude mice, whereas supplement of fish oil components, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) exerts suppressive effects. Fish oil has been shown to reduce the induction of different cancer in animal models by a mechanism which may involve suppression of mitosis, increased apoptosis through long-chain n-3 polyunsaturated fatty acid EPA[1,2,4-6]. In parallel, dietary supplementation with DHA is accompanied by reduced levels of 12- and 15-hydroxyeicosatetraenoic acids (12- and 15-HETE), suggesting that changes in eicosanoid biosynthesis may have been responsible for the observed decrease in tumor growth[2,7-9]. Previous studies also have shown that the anti-cancer effect of fish oil is accompanied by a decreased production of cyclooxygenase and lipoxygenase metabolites[10-13]. The efficacy of fish oil which we have found exhibits particularly potent anticancer effects that appear to be related to its content of lipoxygenase inhibitors rather than its EPA or DHA contents.
Oil A is lipid isolates from the epithelial layer of the echinoderm. Little attention of the mechanism of apoptosis induced by oil A has been paid to pancreatic cancer, which is a major cause of cancer death. The results from the present study indicate that oil A induces apoptosis on pancreatic cancer cells via caspase activities, release of cytochrome c from mitochondria, decreased expression of Bcl-2 and Mcl-1 proteins, increased expression of Bax, redistribution of cell cycle phases, increased expression of P21, GADD45 and GADD153, in association with different expression of cyclins[14-21].
Caspases are cysteine proteases produced as inactive forms and activated during apoptosis. Caspase 3 is a major executioner protease, responsible for initiating in the apoptotic program[22-24]. Caspase 3 plays an important role in the apoptotic program of cells[22-28]. As a result, the caspase 3 and caspase 9 were activated, caspase 7 was decreased following oil A treatment. As a major executioner protease, the sequence alignment among caspase 3, 7 and 9 reveals the different structural basis of functions of caspase 3, 7 and 9. Caspase 3 and caspase 7 share the same sequence by 54% and the backbone structures are nearly identical[29]. The activation of an effector, caspase 3, is performed by an initiator, caspase 9 through proteolytic cleavage at specific internal asp residues and caspase 3 is responsible for initiating the apoptotic program[22-24,30,31].
PARP cleavage could serve as a sensitive parameter for identification of early apoptosis[32,33]. In parallel, PARP provided further evidence that this apoptotic pathway is activated.
Oil A induced cytochrome c release from the mitochondria to cytosol. Once cytochrome c is released from the mitochondria, it complexes with apoptotic protease-activating factor 1 to activate caspase 3[34-37].
Also, the increase of Bax and the decrease of Bcl-2 and Mcl-1 indicate that Bcl proteins-mitochondria-caspase cascade play an important role in oil-induced apoptosis. To better understand the effect of oil A on apoptosis of pancreatic cancer cells, flow cytometric analysis of propidium iodide-stained cells was carried out. Cytometric analysis showed significant changes of cell cycle distribution in MiaPaCa-2 and AsPC-1 cells following oil treatment for 24 hours. At first (control), the proportion of cells was arrested in the G0/G1. The G2/M phase and S phase of cell cycle were accumulated after oil treatment. In the present study, we demonstrated that the distribution of cell cycle underwent changes from G0/G1 phase arrest to G2/M phase and S phase arrest following oil A treatment. The changes of cell cycle distribution suggest that apoptosis of pancreatic cancer cells may be related to some underlying mechanism, which may trigger an apoptosis signal. Thereafter, the cells with a sub-G0/G1 DNA content, a hallmark of apoptosis, were accumulated in both cell lines MiaPaCa-2 and AsPC-1 at 24 hours following exposure to oil A. The effects of oil A persist throughout the course of cell growth, cells with DNA damage increase progressively and develop apoptosis successively. The typical peak of a sub-G0/G1 population of cells indicates oil-induced apoptosis of pancreatic cancer cells.
The cyclins and cyclin-dependent kinases (CDKs) are key components of the cell cycle machinery that is responsible for the progression through the G1/S and G2/M phases[38]. In our experiments, the differential expression of cyclins and CDKs were observed in pancreatic cancer cells. During oil A treatment, no detectable changes of CDK2, 4, 6 were observed. When the cell cycle procession was arrested in G0/G1 phase at 24 hours of treatment, the expression of cyclins remained unchanged. Thereafter, when the cell cycle procession was arrested in G2/M phase at 24 hours of treatment, expression of cyclins was different between MiaPaCa-2 and AsPC-1 cells. Cyclin A and cyclin B1 levels were slightly decreased and cyclin D1 was markedly decreased in MiaPaCa-2 cells, whereas cyclin A and cyclin B1 levels were markedly decreased and cyclin D1 was slightly decreased in AsPC-1 cells. At the same time, expression of cyclin E was still constantly expressed. These changes of cyclins suggested underlying mechanism of cell cycle distribution in pancreatic cancer cells response to oil stress. How the cyclins are regulated remains unclear.
It has been reported that P21 mediated a significant response to DNA-damaging agents[39,40]. The results show that oil induced an increased expression of P21, whereas the expression of P27 protein was not changed following oil treatment in the present work. These results suggested that P21 protein may play a key role in G0/G1 to G2/M phase arrest leading to apoptosis of pancreatic cancer cells.
The cell cycle is regulated by inhibitory proteins, such as GADDs, which are responsible for the maintenance of the cell cycle checkpoint that prevents inappropriate mitosis[41-44]. GADDs expression played an important role in signal transduction pathway in response to DNA damage[34,42-45]. Pancreatic cancer cells might respond to oil stress by activating signal transduction leading to cell cycle arrest. As an extracellullar stress signal, oil A induced expression of GADDs in pancreatic cancer cells, which may be related to cell cycle arrest through G0/G1 to G2/M phases. The experimental findings suggest that GADD expression may modulate the sensitivity to oil-induced DNA damage leading to apoptosis. The GADD45 is considered to be functionally p53-dependent or p53-independent and GADD153 to be p53-independent. The increase in GADD45 and GADD153 along with a decrease in Bcl-2 and Mcl-1 were seen in pancreatic cancer cells that underwent growth arrest and apoptosis, which may provide clues concerning the mechanism of the effects of oil A. Although GADD expression with cell cycle redistributions are responsive to oil stress, the underlying mechanisms remain obscure. Further studies are required to elucidate how oil modulates the GADD proteins expression.