Published online Dec 15, 2003. doi: 10.3748/wjg.v9.i12.2701
Revised: September 11, 2003
Accepted: October 23, 2003
Published online: December 15, 2003
AIM: The interaction of mucosal addressin cell adhesion molecule 1 (MAdCAM-1) with integrin α4β7 mediates lymphocyte recruitment into mucosa-associated lymphoid tissue (MALT). Nodular gastritis is characterized by a unique military pattern on endoscopy representing increased numbers of lymphoid follicles with germinal center, strongly associated with H pylori infection. The purpose of this study was to address the implication of the MAdCAM-1/integrin β7 pathway in NG.
METHODS: We studied 17 patients with NG and H pylori infection and 19 H pylori-positive and 14 H pylori-negative controls. A biopsy sample was taken from the antrum and snap-frozen for immunohistochemical analysis of MAdCAM-1 and integrin β7. In simultaneous viewing of serial sections, the percentage of MAdCAM-1-positive to von Willebrand factor-positive vessels was calculated. We also performed immunostaining with anti-CD20, CD4, CD8 and CD68 antibodies to determine the lymphocyte subsets co-expressing integrin β7.
RESULTS: Vascular endothelial MAdCAM-1 expression was more enhanced in gastric mucosa with than without H pylori infection. Of note, the percentages of MAdCAM-1-positive vessels were significantly higher in the lamina propria of NG patients than in H pylori-positive controls. Strong expression of MAdCAM-1 was identified adjacent to lymphoid follicles and dense lymphoid aggregates. Integrin β7-expressing mononuclear cells, mainly composed of CD20 and CD4 lymphocytes, were associated with vessels lined with MAdCAM-1-expressing endothelium.
CONCLUSION: Our results suggest that the MAdCAM-1/ integrin α4β7 homing system may participate in gastric inflammation in response to H pylori-infection and contributes to MALT formation, typically leading to the development of NG.
- Citation: Ohara H, Isomoto H, Wen CY, Ejima C, Murata M, Miyazaki M, Takeshima F, Mizuta Y, Murata I, Koji T, Nagura H, Kohno S. Expression of mucosal addressin cell adhesion molecule 1 on vessel endothelium of gastric mucosa in patients with nodular gastritis. World J Gastroenterol 2003; 9(12): 2701-2705
- URL: https://www.wjgnet.com/1007-9327/full/v9/i12/2701.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i12.2701
Lymphocyte recruitment to inflammatory sites is regulated by differential expression of cell surface homing receptors and their interactions with relevant vascular adhesion molecules[1]. An immunoglobulin super family, single-chain 60-kDa glycoprotein, mucosal addressin cell adhesion molecule 1 (MAdCAM-1) is selectively expressed on the endothelium of high endothelial venules in gut-associated lymphoid tissues and vascular endothelial cells in the lamina propria of the small and large intestine[2]. Engagement of MAdCAM-1 to its exclusive ligand, integrin α4β7, on lymphocytes represents a tissue-specific homing mechanism for the intestine and gut-associated lymphoid tissue[2].
Helicobacter pylori causes one of the most common chronic infections in humans[3]. Although H pylori is a noninvasive pathogen[4], persistent infection causes chronic gastritis, which predisposes the mucosa to peptic ulceration, and is thought to be eventually linked to gastric cancer and primary gastric lymphoma, especially the lymphoma of mucosa-associated lymphoid tissue (MALT) type[5,6]. The histopathological features of H pylori-associated gastritis include intense infiltration of granulocytes and lymphocytes[7] and the formation of organized lymphoid follicles[8]. In this regard, nodular gastritis (NG), which is a unique military pattern resembling “goose flesh” on endoscopy[9], is characterized by increased numbers of lymphoid follicles with germinal center[10], in close association with H pylori infection[9,10]. Although NG frequently occurs in children, there are ample evidences at present suggesting that NG is not so uncommon in adults, especially in pre-menopausal women[9,10].
Recently, Hatanaka et al[11] reported that expression of vascular endothelial MAdCAM-1 was enhanced in murine chronic gastritis induced by H pylori. Furthermore, Dogan et al[12] demonstrated strong expression of MAdCAM-1 protein on the vasculature in patients with H pylori-associated MALT type lymphoma. However, little is known about the implication of such adhesion molecules in the pathogenesis of NG. In this study, we investigated MAdCAM-1 expression on vessel endothelium in gastric mucosa of patients with NG by immunohistochemistry, along with the interaction with integrin α4β7. This could shed light on the mechanism of MALT organization in gastric mucosa in response to H pylori infection.
We studied 17 patients who underwent upper gastrointestinal endoscopy for dyspepsia and were diagnosed as having NG between April 1999 and March 2002. They included 2 men and 15 women, ranging 24 to 58 years old (mean, 43 years). As a control, age- and sex- matched 33 subjects with non-ulcer dyspepsia during the same period were recruited in the study. They consisted of 19 H pylori-infected patients without nodular gastritis (H pylori-positive controls) and 14 H pylori-negative ones. None of the control subjects had been treated with non-steroidal anti-inflammatory drugs, proton pump inhibitors, antibiotics, or bismuth compounds during the 4-week period prior to the present study. There were no differences in baseline characteristics such as alcohol intake, current tobacco use and body mass index among the three groups.
At endoscopy, one biopsy specimen was obtained from the antrum within 2 cm of the pyloric ring along the greater curvature. The sample was snap-frozen in OCT compound (Tissue-tek; Miles Inc., Elkhart, IN) in ethanol-dry ice bath and stored at -80 °C until use.
H pylori status was assessed by serology (anti-H pylori immunoglobulin G antibody, HEL-p TEST, AMRAD Co., Melbourne, Australia), rapid urease test (CLO test; Delta West Co., Bentley, Australia) using additional biopsy specimens obtained during endoscopy from the antrum within 2 cm of the pyloric ring and the corpus along the greater curvature. Patients were considered positive for H pylori infection when at least one examination yielded positive results. On the other hand, patients were defined as H pylori-negative if all test results were negative.
MAdCAM-1 protein expression on vascular endothelial cells was studied in situ by immunohistochemistry with the streptavidin-biotin-peroxidase-complex method (Histofine SAB-PO kit, Nichirei Co., Tokyo), as described previously[13]. In brief, frozen tissues were cut into 4-μm thick sections and placed on glass slides coated with 3-aminopropyltriethoxysilane (Dako Co., Glostrup, Denmark). The following steps were performed at room temperature unless otherwise specified. Sections were fixed in 4% paraformaldehyde (Merck Co., Darmstadt, Germany) in phosphate-buffered saline (PBS, pH 7.4) for 20 minutes. After a brief washing in PBS, endogenous peroxidase activity was inhibited for 30 min with methanol containing 0.3% H2O2. Sections were reacted for 20 min with 10% normal rabbit serum (Nichirei Co.) to prevent non-specific binding, and incubated with anti-MAdCAM-1 mouse monoclonal antibody (clone 1G2)[14] at a concentration of 1:100 in PBS overnight at 4 °C. On the next day, the sections were washed three times (10 min each) in PBS and incubated for 20 min with 10 mg/ml biotinylated rabbit anti-mouse immunoglobulins (Nichirei). After washed three times (10 min each) in PBS, the sections were re-incubated for 20 min with 100 µg/ml HRP-conjugated streptavidin (Nichirei). After washed three times (10 min each) in PBS, a color reaction was performed with 0.05 M Tris-HCl (pH 7.6) containing 3, 3’-diaminobendizine tetrahydrochloride (Dojin Chemical Co., Kumamoto, Japan) and H2O2. Sections were counterstained with Mayer’s hematoxylin and then dehydrated, cleared and mounted by standard procedures.
In each serial section of the same tissue specimen, vessels were immunostained with anti-von Willebrand factor monoclonal antibody (Dako Co.) in the same fashion described above, in order to assess quantitatively the difference in the extent of expression of endothelial adhesion molecules, based on the method reported by Hatz et al[15]. Briefly, two independent observers who were blind to the diagnosis and experimental results, counted the number of von Willebrand factor-positive vessels and then the number of MAdCAM-l- positive vessels on the section serial to that stained for von Willebrand factor. Percentage of the ratio of MAdCAM-1-positive to von Willebrand factor-positive vessels was calculated. As a negative control, each non-specific isotype antibody was used instead of the primary antibodies, or the primary antibodies were omitted. When the interobserver variability exceeded 10%, the areas were simultaneously re-evaluated by the two investigators to reach a consensus.
Furthermore, immunoreactivity for integrin β7 on inflammatory cells infiltrating the gastric mucosa was similarly analyzed using a specific monoclonal antibody (Pharmingen Co., San Diego, CA) at a concentration of 1:10. On the section serial to that stained for β7, we also performed immunohistochemistry using anti-CD20, -CD4, -CD8 and -CD68 monoclonal antibodies (purchased from Dako Co.).
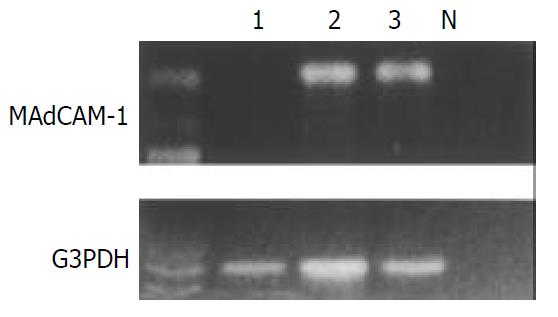
Total RNA from biopsy samples that were also collected from the same location was extracted using a commercial kit according to the instructions provided by the supplier (ISOGEN, Nippon Gene Co., Toyama, Japan). Equivalent amounts of RNA were monitored by absorption at 260 nm and by monitoring the density of 28S and 18S RNA detected after electrophoresis. After 1 µg of total RNA was reverse transcribed to complementary DNA, the target sequence of MAdCAM-1 was amplified in 35 cycles, each consisting of 1 min at 94 °C for denaturation, 1 min at 60 °C for annealing and 1 min at 72 °C for extension, followed by a final extension for 5 min at 72 °C with specific primers[16], using a RT-PCR kit (Takara Shuzo Co., Otsu, Japan). Two primers designed to nucleotide positions 978-999 (TGCGGTGCTGGGACTGCTGCTC, sense) and 1344-1364 (TCAGGGAGGGGCTTCAGGTCA, antisense) of human MAdCAM-1 cDNA sequence were used for amplification of a 387 bp product[16]. A 10 µl aliquot of each PCR product was analyzed by electrophoresis on 2% agarose gel containing ethidium bromide, and the bands were examined under ultraviolet light for the presence of amplified DNA. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) gene transcript was routinely amplified as described previously[17] and used as an internal control of the processed RNA for each preparation.
All samples were obtained with informed consent in accordance with the Helsinki Declaration.
Statistical analyses were performed using Fisher’s exact, χ2, Student’s t, and Mann-Whitney U tests, whichever appropriate. A P value less than 0.05 was accepted as statistically significant. Data were expressed as mean ± standard deviation (SD).
All 17 patients with NG were H pylori-positive. Among the 17 patients, no less than 9 (52.9%) had endoscopic evidence of peptic ulcer (duodenal ulcer in 7 and gastric ulcer in 2). All except one specimen from the NG patients showed lymphoid follicle formation and/or dense lymphoid aggregates.
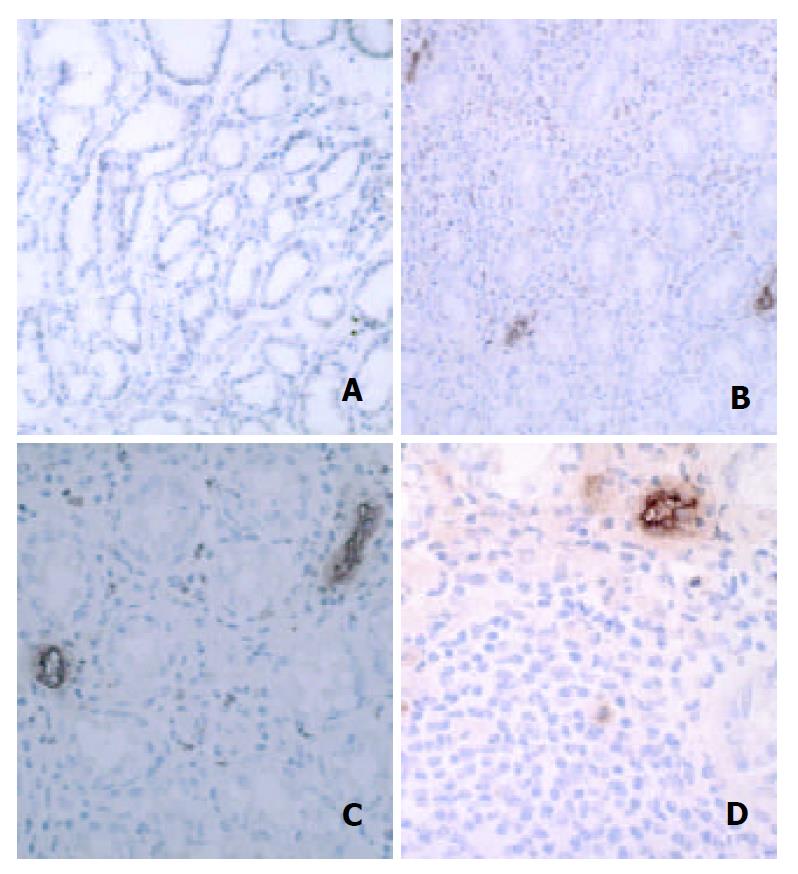
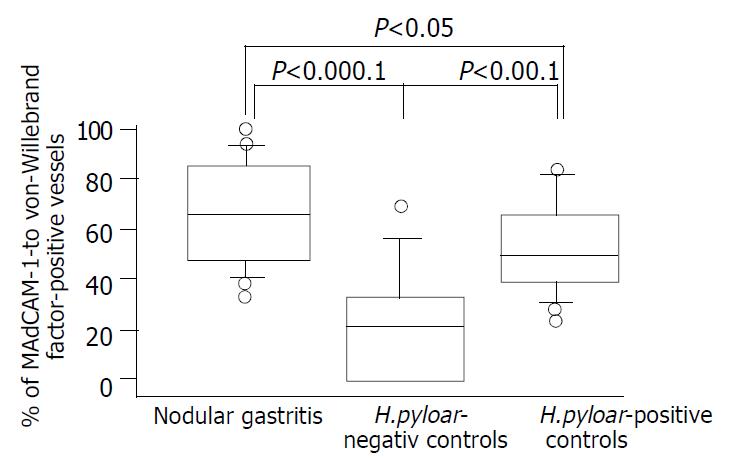
The MAdCAM-1antigen, recognized by the 1G2 antibody, was seen on vascular endothelial cells in gastric mucosa. In H pylori-negative group, MAdCAM-1 was rarely or occasionally localized in vascular endothelial cells in the lamina propria (Figure 1A). However, the relative endothelial area expressing MAdCAM-1 increased in inflammatory settings induced by H pylori infection (Figure 1B), particularly in association with dense lymphocytic infiltration (Figure 1C). High endothelial venule-like vessels adjacent to the lymphoid follicle were intensely immunoreactive (Figure 1D). There was a significant difference in the percentages of MAdCAM-l-positive vessels between H pylori-positive and –negative controls (P < 0.001, Figure 2). In addition, the expression of vascular MAdCAM-1 was more enhanced in patients with NG than that in H pylori-negative controls (P < 0.0001, Figure 2). Of note, MAdCAM-1-positive vessels were more abundant in the antral mucosa of patients with NG, compared with H pylori-positive controls (P < 0.05, Figure 2). The percentages of MAdCAM-1-positive vessels detected by the two observers were almost identical. When each non-specific isotype serum was used or primary antibodies were omitted, the specimens showed no immunoreactivity.
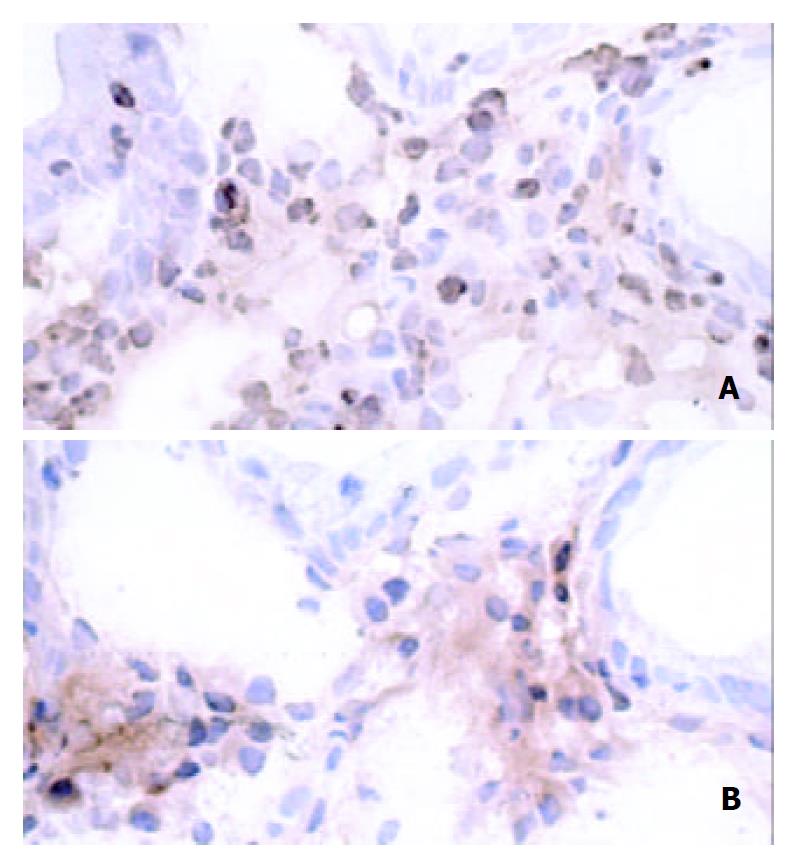
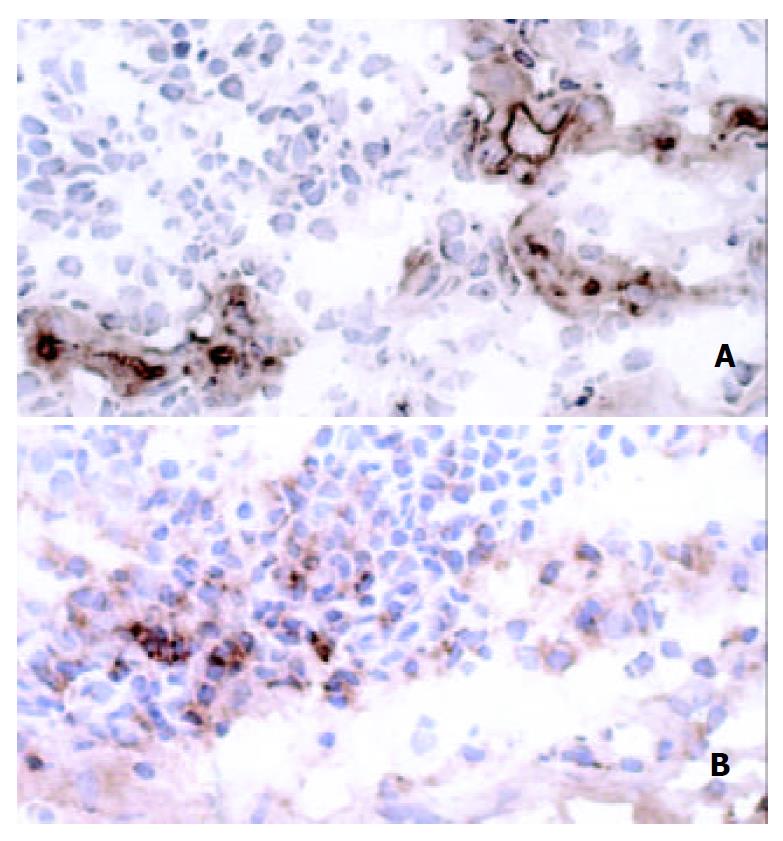
Integrin β7 was expressed on increased number of mononuclear cells in the lamina propria of patients with H pylori infection, whereas there were few integrin β7-positive cells in the mucosa of H pylori-negative controls. Analysis of serial sections showed lymphocytes co-expressing anti-CD4 antibody and integrin β7 (Figure 3). Also, some CD20-positive cells also expressed integrin β7. However, the integrin β7-positive infiltrating cells showed little immunoreactivity for CD8 and CD68. The vessels lined with endothelial cells positive for MAdCAM-1 correlated well with the infiltration of integrin β7-expressing lymphocytes (Figure 4).
We identified the MAdCAM-1 gene-specific product as a 387-bp band by RT-PCR both in patients with NG and in H pylori-positive controls, but not in H pylori-negative controls despite the constitutive G3PDH housekeeping gene expression (Figure 5).
In the present study, vascular expression of MAdCAM-1 protein assessed by immunohistochemistry was more enhanced in gastric mucosa of H pylori-positive than in H pylori–negative subjects. On RT-PCR analysis, the mRNA for MAdCAM-l was detected only in H pylori-infected gastric mucosa, but not in the mucosa negative for the organism. In the previous in vitro study, MAdCAM-1 was induced on a murine endothelial cell line, by proinflammatory cytokines including interleukin 1 and tumor necrosis factor α[18], which were much more increased in human gastric mucosa with than without H pylori infection[19]. Considered together, these results indicate that MAdCAM-1 is selectively upregulated in the infected gastric mucosa. Integrin β7-expressing mononuclear cells were found surrounding blood vessels lined with MadCAM-1-postive endothelium, suggesting that the MAdCAM-1/integrin α4β7 pathway directs lymphocytes to the inflamed sites of H pylori infection, as shown in the murine model[11]. Together with other endothelially expressed adhesion molecules such as intercellular cell adhesion molecule 1 and vascular cell adhesion molecule 1[15], though more specifically, MAdCAM-1 could play a role in chronic inflammation induced by H pylori.
The striking finding of this study was that the percentages of MAdCAM-1-positive to von Willebrand factor-positive vessels in the lamina propria was significantly higher in patients with NG than in H pylori-positive controls. In addition to the prominent development of lymphoid follicles, intense mucosal inflammatory cell infiltration consisting mainly of lymphocytes was characteristic of NG[10]. The recruitment and emigration of circulating lymphocytes, on which integrin α4β7 is certainly expressed[20], may be accelerated in the gastric mucosa of NG, through, in part, the substantially enhanced expression of this vascular addressin.
MAdCAM-1 protein was strongly expressed on high endothelial venule-like vessels adjacent to lymphoid follicles and dense lymphoid aggregates within gastric mucosa. In the gut-associated lymphoid tissues such as Peyer’s patches and mesenteric lymph nodes, the homing of their constituent lymphocytes has been found to be largely dependent on the interaction of MAdCAM-1 on high endothelial venules with integrin α4β7[2]. Similarly, the vascular MAdCAM-1 localized near the lymphoid follicles may be a principal ligand for circulating lymphocytes to the specialized lymphoid sites and may contribute to the formation of MALT in H pylori-induced gastric inflammation, typically leading to the development of NG. This is a topic that warrants further studies, with special attention to the relationship between NG and MALT lymphoma[21], as a recent report documented the strong expression of MAdCAM-1 on the vasculature of patients with this type of lymphoma[12].
In the present study, simultaneous viewing of serial sections revealed the presence of integrin β7-expressing mononuclear cells in the infected gastric mucosa. These cells were mainly CD4-positive T and CD20-positive B cell populations, as described previously[12,20,22]. Several in vitro studies showed that H pylori antigen could induce the expression of integrin α4β7 on the surface of these cells[20,22]. In addition, employing immunomagnetic cell sorting, H pylori reactive circulating T lymphocytes were mainly found in the α4β7-positive cell fraction[20]. Accordingly, we believe that mucosally activated lymphocytes in response to H pylori infection can migrate from the inductive sites via circulation to their effector sites in the gastric mucosa, using this exclusive homing receptor as a ligand for MAdCAM-1.
In conclusion, our study demonstrated enhanced expression of MAdCAM-1 on the vascular endothelium and increased number of integrin β7-expressing T and B cells in H pylori-positive gastric mucosa. In NG, the proportion of MAdCAM-1-positive vessels was higher than that even in H pylori-positive controls. The selective MAdCAM-1/integrin α4β7 homing system is thought to play a significant role in gastric mucosal immunity in response to H pylori infection, especially in lymphoid follicle formation.
Edited by Wang XL
| 1. | Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5269] [Cited by in RCA: 5167] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 2. | Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97-110. [PubMed] |
| 3. | Dytoc M, Gold B, Louie M, Huesca M, Fedorko L, Crowe S, Lingwood C, Brunton J, Sherman P. Comparison of Helicobacter pylori and attaching-effacing Escherichia coli adhesion to eukaryotic cells. Infect Immun. 1993;61:448-456. [PubMed] |
| 4. | Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1997;11 Suppl 1:71-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 5. | Weber DM, Dimopoulos MA, Anandu DP, Pugh WC, Steinbach G. Regression of gastric lymphoma of mucosa-associated lymphoid tissue with antibiotic therapy for Helicobacter pylori. Gastroenterology. 1994;107:1835-1838. [PubMed] |
| 6. | Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 478] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Meining A, Stolte M, Hatz R, Lehn N, Miehlke S, Morgner A, Bayerdörffer E. Differing degree and distribution of gastritis in Helicobacter pylori-associated diseases. Virchows Arch. 1997;431:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Genta RM, Hamner HW, Graham DY. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993;24:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 250] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Miyamoto M, Haruma K, Yoshihara M, Hiyama T, Sumioka M, Nishisaka T, Tanaka S, Chayama K. Nodular gastritis in adults is caused by Helicobacter pylori infection. Dig Dis Sci. 2003;48:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | De Giacomo C, Fiocca R, Villani L, Lisato L, Licardi G, Diegoli N, Donadini A, Maggiore G. Helicobacter pylori infection and chronic gastritis: clinical, serological, and histologic correlations in children treated with amoxicillin and colloidal bismuth subcitrate. J Pediatr Gastroenterol Nutr. 1990;11:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 78] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Hatanaka K, Hokari R, Matsuzaki K, Kato S, Kawaguchi A, Nagao S, Suzuki H, Miyazaki K, Sekizuka E, Nagata H. Increased expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and lymphocyte recruitment in murine gastritis induced by Helicobacter pylori. Clin Exp Immunol. 2002;130:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Dogan A, Du M, Koulis A, Briskin MJ, Isaacson PG. Expression of lymphocyte homing receptors and vascular addressins in low-grade gastric B-cell lymphomas of mucosa-associated lymphoid tissue. Am J Pathol. 1997;151:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Isomoto H, Furusu H, Shin M, Ohnita K, Miyazaki M, Omagari K, Mizuta Y, Murase K, Inoue K, Murata I. Enhanced expression of transcription factor E2F in Helicobacter pylori-infected gastric mucosa. Helicobacter. 2002;7:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Arihiro S, Ohtani H, Suzuki M, Murata M, Ejima C, Oki M, Kinouchi Y, Fukushima K, Sasaki I, Nakamura S. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn's disease. Pathol Int. 2002;52:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Hatz RA, Rieder G, Stolte M, Bayerdörffer E, Meimarakis G, Schildberg FW, Enders G. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology. 1997;112:1908-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Leung E, Berg RW, Langley R, Greene J, Raymond LA, Augustus M, Ni J, Carter KC, Spurr N, Choo KH. Genomic organization, chromosomal mapping, and analysis of the 5' promoter region of the human MAdCAM-1 gene. Immunogenetics. 1997;46:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Ikeda K, Oka M, Yamada Y, Soda H, Fukuda M, Kinoshita A, Tsukamoto K, Noguchi Y, Isomoto H, Takeshima F. Adult T-cell leukemia cells over-express the multidrug-resistance-protein (MRP) and lung-resistance-protein (LRP) genes. Int J Cancer. 1999;82:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Takeuchi M, Baichwal VR. Induction of the gene encoding mucosal vascular addressin cell adhesion molecule 1 by tumor necrosis factor alpha is mediated by NF-kappa B proteins. Proc Natl Acad Sci U S A. 1995;92:3561-3565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 281] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Quiding-Järbrink M, Ahlstedt I, Lindholm C, Johansson EL, Lönroth H. Homing commitment of lymphocytes activated in the human gastric and intestinal mucosa. Gut. 2001;49:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Miyamoto M, Haruma K, Hiyama T, Kamada T, Masuda H, Shimamoto F, Inoue K, Chayama K. High incidence of B-cell monoclonality in follicular gastritis: a possible association between follicular gastritis and MALT lymphoma. Virchows Arch. 2002;440:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Barrett SP, Riordon A, Toh BH, Gleeson PA, van Driel IR. Homing and adhesion molecules in autoimmune gastritis. J Leukoc Biol. 2000;67:169-173. [PubMed] |