Published online Nov 15, 2003. doi: 10.3748/wjg.v9.i11.2552
Revised: May 12, 2003
Accepted: May 19, 2003
Published online: November 15, 2003
AIM: To explore the correlation of magnifying endoscopic patterns and histopathology, Helicobacter pylori (H. pylori) infection of the gastric mucosa.
METHODS: Gastric mucosal patterns in 140 patients with chronic gastritis were studied using Olympus GIF-Q240Z magnifying endoscope. Histopathological examination, rapid urease test and Warrthin-Starry staining were taken with biopsy samples from the magnified sites of stomach. The magnifying endoscopic patterns were compared with histopathological results and H. pylori detection.
RESULTS: The pit patterns of gastric mucosa were classified as types A (round spot), B (short rod), C (branched), D (reticular) and E (villus). The detection rate of chronic atrophic gastritis (CAG) by magnifying endoscopy was 94.3% (33/35), which was significantly higher than that by routine endoscopy (22.9%, 8/35) (P < 0.01). The pit patterns of 31 cases of intestinal metaplasia (IM) appeared as type E in 18 cases (58.1%), type D in 8 cases (25.8%) and type C in 5 cases (16.1%). Fourteen out of 18 patients (77.8%) with complete type (type I) of IM appeared as type E of pit patterns, whereas only 4 of 13 (30.8%) patients with incomplete type (types II and III) of IM appeared as type E (P < 0.05). Collecting venules in the anterior of lower part of gastric corpus were subgrouped into types R (regular), I (irregular) and D (disappeared). H. pylori infection was found in 12.2% (9/74), 60% (9/15) and 84.3% (43/51) cases in these types respectively. H. pylori infection rate in type R was significantly lower than that in other two types (P < 0.01).
CONCLUSION: Magnifying endoscopy may have an obvious value in diagnosing chronic atrophic gastritis, intestinal metaplasia and H. pylori infection.
- Citation: Yang JM, Chen L, Fan YL, Li XH, Yu X, Fang DC. Endoscopic patterns of gastric mucosa and its clinicopathological significance. World J Gastroenterol 2003; 9(11): 2552-2556
- URL: https://www.wjgnet.com/1007-9327/full/v9/i11/2552.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i11.2552
Recently, magnifying endoscope has been used clinically for its developments in amplifying power, definition and operational capability. Lots of international studies on clinical application of magnifying endoscope especially from Japan have been reported, but most of them were focused on colon and esophagus, only a few of them on gastric mucosa have been published[1-28]. These studies implicate that classification of superficial mucosal appearances defined by magnifying endoscopy can reflect not only histological features but also mucin phenotypes. Magnifying endoscopy is helpful for more correctly distinguishing hyperplastic lesions from adenomatous and cancerous lesions, and for improving detection of early flat and depressed cancer. More interestingly, according to Japanese data, magnifying endoscopy could also be used to predict invasive depth and lymph node metastasis of cancer[6,16]. Up to now only a few studies on the field have been reported in China[29-33]. In this article, we reported our study on correlation of magnifying endoscopic patterns and histopathology, Helicobacter pylori (H. pylori) infection of gastric mucosa in 140 patients with chronic gastritis to understand the value of magnifying endoscopy in diagnosing the minute lesions of gastric mucosa.
Subjects were 140 out-patients and in-patients (male: 68, female: 72, age range: 18-77 years old, average age: 50.6) with chronic superficial gastritis (CSG, n = 105) and chronic atrophic gastritis (CAG, n = 35) during June-August, 2002. All the patients had gastrointestinal symptoms such as abdominal distention, abdominalgia, belch and hyperhydrochloria.
New model of electronic magnifying endoscope GIF Q-240Z (Olympus Optical Co., Ltd., Tokyo, Japan) was used. It could be used to perform routine endoscopy (observation at standard magnification) as well as to magnify the image 80 times (in 14-inch monitor) as large as the original size through manual adjustment of the focal length.
Magnifying endoscopy was performed by senior endoscopists and the real time static and successive images were recorded by computer image and text reporting system and video tape recorder. In order to inhibit gastrointestinal peristalsis, 10 mg of anisodaminum (654-2) and 5-10 mg of diazepamum were injected intramuscularly at 10 min pre-endoscopy. Routine endoscopy was performed first, and if necessary, dilution of dimethyl silicone oil was used to flush off the foam and mucus, then the appearance of gastric pits in the antrum, angel, corpus and fundus, and collecting venules in the anterior of lower part of gastric corpus were observed so that the patterns of gastric pits and collecting venules could be decided.
One piece of tissue in the greater curvature of gastric antrum was extracted for rapid urease test using RUT kit (Kedi Technology Co., LTD, Zhuhai, China). Two biopsies from the magnified sites in gastric antrum and corpus were performed for hematoxylin-eosin (HE) and Warrthin-Starry staining. Rapid urease test and Warrthin-Starry staining were used for H. pylori detection[34,35]. According to the national standard of China, H. pylori infection was established after positive results were confirmed by both rapid urease test and Warrthin-Starry staining[35]. HE staining was performed for routine histopathological examination. Inflammatory levels were graded as mild, moderate and severe types according to the infiltration depth of < 1/3, 1/3-2/3 and > 2/3 of inflammatory cells in the mucous layer and CAG was graded as mild, moderate and severe types according to the decreased levels of < 1/3, 1/3-2/3 and > 2/3 of the intrinsic glands[35]. At the same time, mucus histochemical stainings of AB/PAS for distinguishing acid mucus from neutral mucus and AF/AB for distinguishing sulphomucins from sialomucins were performed on the samples with IM confirmed by histopathological examination. According to the histological structures and properties of mucus excreted by cells, IM was classified into type I (complete type), type II (incomplete small intestinal type) and type III (incomplete colonic type)[36].
χ² test was applied and P values less than 0.05 were considered significant.
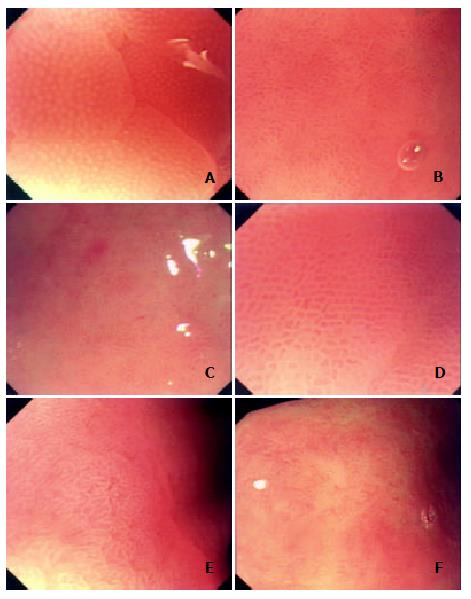
Classification of gastric pits Based on the analysis of recorded static and successive images and refered to Guelrud's study on mucosal patterns of Barrett's esophagus with enhanced magnification endoscopy[18], we classified gastric pits into the following five fundamental types: type A, round-spot-like, distributing only in the gastric corpus and fundus with basically normal histology; type B, short-rod-like, with deeper pits, branches and curvatures fewer than those in type C, mainly distributing in the gastric antrum without obvious lesions such as inflammation; type C, with elongated and tortuous pits with obviously increased branches and curvatures, connected to present branch-like form, seen in mucosa with pathological changes as inflammation, edema and IM; type D, with reticular pits, seen in areas with more severe inflammation, edema and IM, also found in mucosa around erosion and ulcers; and type E, with villus-like pits, or with finger-like tubers, similar to enteral villus-like changes, seen only in the areas with IM (Figure 1). Overlappings and crossings of gastric pits might be present except the above five fundamental patterns. For example, a combination of type A and type B of gastric pits was frequently presented in the gastric angle.
In types B, C, D and E, moderate and severe inflammations were seen in 18.6% (26/140), 85.1% (40/47), 100% (13/13) and 88.9% (16/18) cases respectively. The inflammatory levels in types C, D and E were significantly higher than those in type B (P < 0.01).
Features of atrophic gastritis under magnifying endoscope Under routine endoscope, changes of rough mucosa, unflat granules, increased white areas, exposure of submucous vessels could be seen in CAG[35]. Generally, theses changes could be more easily found in severe CAG, but it was hard to identify them in mild CAG. However, under magnifying endoscope, remarkably characteristic changes of CAG could be seen. With a comparatively low magnifying power, obvious red-white mucosa and increased white areas could be identified. When with enhanced magnifying power, disordered structures, decrease in quantity and even disappearance of pits as scar-like change could be observed in white areas (Figure 1F).
Thirty-five patients with different grades of gastric mucous atrophy were confirmed by pathological examination in 140 patients, in which 27 in gastric antrum, 5 in gastric angle, 3 in gastric corpus, 16 with mild atrophy, 7 with moderate atrophy and 12 with severe atrophy. By routine endoscopy, only 8 patients (2 with moderate atrophy, 6 with severe atrophy), but by magnifying endoscopy, CAG-related changes were found in 33 patients (14 with mild atrophy, 7 with moderate atrophy, 12 with severe atrophy). The detection rates of atrophic gastritis by routine endoscopy and magnifying endoscopy were 22.9% (8/35) and 94.3% (33/35) respectively and significant difference was found by comparison of the two kinds of endoscopy (P < 0.01).
Features of intestinal metaplasia under magnifying endoscope It was reported that characteristic changes such as light yellow or ivory-white nodosity-like, fishscale-like and diffusing granule-like appearances of IM in gastric mucosa could be found under routine endoscopy[37]. In this study, by analysis of magnifying endoscopy images of 31 patients with IM, we found that there were mainly three patterns of gastric pits in IM mucosal areas: type C (5 cases), type D (8cases) and type E (18cases). Particularly, very high specificity was found in type E. IM was confirmed pathologically in all the samples of 18 patients with type E. After classification of all the samples with IM by mucous histochemical staining, a certain relation was found between the patterns of gastric pits and classification of IM mucin phenotypes, as was shown in Table 1. Fourteen out of 18 patients (77.8%) with complete type (type I) of IM appeared as type E of pit patterns, whereas only 4 of 13 (30.8%) patients with incomplete type (types II and III) of IM appeared as the same type of pit patterns (P < 0.05).
| Pit patterns | Cases | Type I IM | Type II IM | Type III IM |
| Type C | 5 | 2 | 3 | 0 |
| Type D | 8 | 2 | 5 | 1 |
| Type E | 18 | 14 | 3 | 1 |
| Total | 31 | 18 | 11 | 2 |
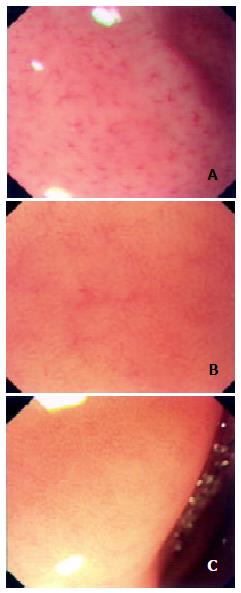
With reference to Yagi's literature[26], we classified the architecture of collecting venules into the following three types: type R (regular type) with diameter of minor venules being 0.4-0.5 mm and regular spider-like and jellyfish-like arrangement, type I (irregular type) in which decrease in quantity of collecting venules could be unclearly found with irregular arrangement, and type D (disappeared) in which collecting venules could not be found under magnifying endoscope (Figure 2).
When the patients in which positive H. pylori was confirmed by both rapid urease test and Warrthin-Starry staining were regarded as being infected by H. pylori, the H. pylori infection rates in the patients with regular (R), irregular (I) and disappeared (D) types of collecting venules were 12.2%, 60% and 84.3%, respectively. Statistical analysis revealed that H. pylori infection rates in the patients with types D and I were markedly higher than those in patients with type R (P < 0.01), but there was no significance between type I and type D (P > 0.05). A comparison of different types of collecting venules by the corresponding rapid urease test and by Warrthin-Starry staining is shown in Table 2.
| Collecting venules | Cases | Rapid urease test (+) | W-S staining (+) | Both methods (+) |
| Type R | 74 | 9 (12.2%) | 13 (17.6%) | 9 (12.2%) |
| Type I | 15 | 11 (73.3%) | 9 (60%) | 9 (60%) |
| Type D | 51 | 43 (84.3%) | 45 (86.3%) | 43 (84.3%) |
The pit patterns observed on the mucosal surface are considered to reflect the arrangement and structure of surface epithelia, morphology, number, distribution, and function of glands, mucosal edema and inflammation, and vascular morphology, arrangement, number and distribution. The basic units of the microstructures on the surface of gastric mucosa are countless gastric pits that form gastric areas separated by minor gastric grooves (also called interval grooves). As the openings of glands, gastric pits are the first to have changes of the structures due to gastric mucosal lesions. Magnifying endoscope can be used to observe the minute architecture of gastric pits because it has the similar magnifying power to that of stereomicroscope.
In this research, we studied the correlation of magnifying endoscopic patterns and histopathology, Helicobacter pylori infection of the gastric mucosa in 140 patients with chronic gastritis. There has been no widely accepted standard to the classification of gastric pits under magnifying endoscope, so the classification method, we used, was based on the analysis of our recorded static and successive images by magnifying endoscopy in the 140 patients and referred to Guelrud's study on mucosal patterns of Barrett's esophagus with enhanced magnification endoscopy[18]. Type A and type B represented the manifestation of normal gastric pits in gastric corpus and antrum, which concerned the distribution of gastric glands. Single tabular glands with short and fine neck were found in the gastric corpus and fundus, so gastric pits were presented with round spots as the openings of the gastric glands. Glands in frontal area of pyloric ostium and in gastric antrum were of multi-branches and curvatures and 3-5 glands often shared the same opening in one pit, thus the pits in frontal area of pyloric ostium and gastric antrum were short rod-like and deeper and longer than those of type A. Types C and D were formed by the enlargement, elongation, tortuosity of pits and connection of pits due to the pathological changes as inflammation and edema but type E might be the characteristic changes of intestinal metaplasia. Studies by Endo et al[15] of the mucosa with intestinal metaplasia in Barret esophagus in gastric cardia also revealed that villus-like pits were the characteristic change due to intestinal metaplasia.
Gastric mucosal atrophy could be identified by routine endoscopy usually when it was at more severe grade. Under magnifying endoscopy, disordered structures, deficiency and even disappearance of gastric pits were of high detection rate and accuracy for atrophic gastritis. As to mild and moderated grades of atrophy, the diagnostic sensitivity by magnifying endoscopy was higher than that by routine endoscopy. The decrease and disappearance of gastric pits due to atrophy were different from mucosal defect due to erosion in which there usually smooth-edged pits belonging to types C and D.
It was reported that characteristic changes such as light yellow or ivory-white nodosity-like, fishscale-like and diffusing granule-like appearances of intestinal metaplsia in gastric mucosa could be found under routine endoscopy[37]. The intestinal metaplasia could be classified into complete and incomplete types according to the histological structures and the properties of mucus excreted by cells[36]. In our study, the pit patterns of 31 patients with intestinal metaplasia appeared as type E in 18 (58.1%), type D in 8 (25.8%) and type C in 5 (16.1%). Fourteen out of 18 patients (77.8%) with complete type (type I) of intestinal metaplasia appeared as villus-like and finger-like changes (type E) of pit patterns, whereas only 4 out of 13 (30.8%) patients with incomplete type (types II and III) of intestinal metaplasia appeared as the same type of pit patterns (P < 0.05), suggesting type E of gastric pits was the result of characteristic change of complete intestinal metaplasia. In addition, our study also reveals that pits of incomplete intestinal metaplasia mainly belonged to types C and D (9/13, 69.2%). Nevertheless, the above studies were still at preliminary stage with a small number of samples and further studies should be conducted to draw the final conclusion.
Collecting venules are tiny venules in gastric mucosa directly connected with capillary vessels. A few reports have been made on the architecture of collecting venules in gastric mucosa by magnifying endoscopy in which it was regarded as having certain specificity and feasibility to detect Helicobacter pylori infection[25,26]. It has also been verified by our study that Helicobacter pylori infection rate of patients with type R collecting venules was significantly lower than that with types I and D, suggesting that magnifying endoscopy was of high value in the diagnosis of Helicobacter pylori infection in gastric mucosa. As to the causes leading to the changes of collecting venules when Helicobacter pylori infection occurs, it has been reported that they were found in types I and D, in which remarkable increase of infiltration of neutrophils and monocytes was found and the architecture of collecting venules might be affected by edema of mucosa due to Helicobacter pylori infection[25,26]. However, further studies of the precise mechanism should be conducted because there are some other causes resulting in edema of mucosa.
In conclusion, it is a novel topic in the field of digestive endoscopy to diagnose minute lesions in gastric mucosa by magnifying endoscopy. Our preliminary study has shown that magnifying endoscopy is of high value in the diagnosis of gastric mucosal atrophy, intestinal metaplasia and Helicobacter pylori infection. However, the pit patterns of gastric mucosa, particularly those under magnifying chromoendoscopy are very complicated and there has been no widely accepted standard on the classification. Therefore, further studies are suggested on the clinicopathological significance of different patterns of gastric pits, particularly the characteristic changes of gastric pits and microvessels of intramucosal gastric carcinomas.
Edited by Xia HHX and Wang XL
| 1. | Makin GB, Breen DJ, Monson JR. The impact of new technology on surgery for colorectal cancer. World J Gastroenterol. 2001;7:612-621. [PubMed] |
| 2. | Peitz U, Malfertheiner P. Chromoendoscopy: from a research tool to clinical progress. Dig Dis. 2002;20:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Tamura S, Furuya Y, Tadokoro T, Higashidani Y, Yokoyama Y, Araki K, Onishi S. Pit pattern and three-dimensional configuration of isolated crypts from the patients with colorectal neoplasm. J Gastroenterol. 2002;37:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Tonooka T, Sano Y, Fujii T, Kato S, Yoshino T, Fu KI, Hironaka S, Ochiai A, Yoshida S. Adenocarcinoma in solitary large hyperplastic polyp diagnosed by magnifying colonoscope: report of a case. Dis Colon Rectum. 2002;45:1407-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Morita T, Tamura S, Miyazaki J, Higashidani Y, Onishi S. Evaluation of endoscopic and histopathological features of serrated adenoma of the colon. Endoscopy. 2001;33:761-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Matsumoto T, Hizawa K, Esaki M, Kurahara K, Mizuno M, Hirakawa K, Yao T, Iida M. Comparison of EUS and magnifying colonoscopy for assessment of small colorectal cancers. Gastrointest Endosc. 2002;56:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Hurlstone DP, Fujii T, Lobo AJ. Early detection of colorectal cancer using high-magnification chromoscopic colonoscopy. Br J Surg. 2002;89:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Tung SY, Wu CS, Su MY. Magnifying colonoscopy in differentiating neoplastic from nonneoplastic colorectal lesions. Am J Gastroenterol. 2001;96:2628-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Kato S, Fujii T, Koba I, Sano Y, Fu KI, Parra-Blanco A, Tajiri H, Yoshida S, Rembacken B. Assessment of colorectal lesions using magnifying colonoscopy and mucosal dye spraying: can significant lesions be distinguished. Endoscopy. 2001;33:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Bruno MJ. Magnification endoscopy, high resolution endoscopy, and chromoscopy; towards a better optical diagnosis. Gut. 2003;52 Suppl 4:iv7-i11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Kudo S, Kashida H, Tamura T, Kogure E, Imai Y, Yamano H, Hart AR. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg. 2000;24:1081-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 252] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Nagata S, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Pit pattern diagnosis of early colorectal carcinoma by magnifying colonoscopy: clinical and histological implications. Int J Oncol. 2000;16:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Inoue H. Magnification endoscopy in the esophagus and stomach. Digestive Endoscopy. 2001;13:S40-S41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Sharma P, Weston AP, Topalovski M, Cherian R, Bhattacharyya A, Sampliner RE. Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett's oesophagus. Gut. 2003;52:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Endo T, Awakawa T, Takahashi H, Arimura Y, Itoh F, Yamashita K, Sasaki S, Yamamoto H, Tang X, Imai K. Classification of Barrett's epithelium by magnifying endoscopy. Gastrointest Endosc. 2002;55:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Kumagai Y, Inoue H, Nagai K, Kawano T, Iwai T. Magnifying endoscopy, stereoscopic microscopy, and the microvascular architecture of superficial esophageal carcinoma. Endoscopy. 2002;34:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Guelrud M, Herrera I, Essenfeld H, Castro J, Antonioli DA. Intestinal metaplasia of the gastric cardia: A prospective study with enhanced magnification endoscopy. Am J Gastroenterol. 2002;97:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Guelrud M, Herrera I, Essenfeld H, Castro J. Enhanced magnification endoscopy: A new technique to identify specialized intestinal metaplasia in Barrett's esophagus. Gastrointest Endosc. 2001;53:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Yao K, Oishi T. Microgastroscopic findings of mucosal microvascular architecture as visualized by magnifying endoscopy. Digestive Endoscopy. 2001;13:S27-S33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Tajiri H, Matsuda K, Fujisaki J. What can see with the endoscope Present status and future perspectives. Digestive Endoscopy. 2002;14:131-137. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Miwa H, Sato N. Shed light again on magnifying endoscopy for diagnosis of early gastric cancer. Digestive Endoscopy. 2001;13:127-128. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Niwa Y, Goto H, Ohmiya N, Ohtsuka Y, Ando N. Magnifying endoscopy for the diagnosis of early gastric cancer. Digestive Endoscopy. 2002;14:S70-S71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 23. | Tajiri H, Doi T, Endo H, Nishina T, Terao T, Hyodo I, Matsuda K, Yagi K. Routine endoscopy using a magnifying endoscope for gastric cancer diagnosis. Endoscopy. 2002;34:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002;34:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Calès P, Oberti F, Delmotte JS, Baslé M, Casa C, Arnaud JP. Gastric mucosal surface in cirrhosis evaluated by magnifying endoscopy and scanning electronic microscopy. Endoscopy. 2000;32:614-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Fujiya M, Saitoh Y, Nomura M, Maemoto A, Fujiya K, Watari J, Ashida T, Ayabe T, Obara T, Kohgo Y. Minute findings by magnifying colonoscopy are useful for the evaluation of ulcerative colitis. Gastrointest Endosc. 2002;56:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Jiang B. Chromoendoscopy and high-magnification colonoscopy in early detection of colorectal cancer. Diyi Junyi Daxue Xuebao. 2002;22:385-387. [PubMed] |
| 30. | Shi HX, Wu YL. Distinguishing colorectal minute lesions by high-resolution video endoscope with indigo carmine dye spray. Zhonghua Xiaohua Neijing Zazhi. 1999;16:135-137. |
| 31. | Li Z, Zhang S, An D, Chen F, Gong J. [Diagnosis and treatment of early colorectal cancer]. Zhonghua Waike Zazhi. 2000;38:352-354, 324. [PubMed] |
| 32. | Yu YZ, Wang QH, Yu ZL. The gastric mucosal features of Helicobacter pylori associated gastritis evaluated byhigh-resolution magnifying endoscopy. Zhonghua Xiaohua Neijing Zazhi. 2002;19:274-277. |
| 33. | Chen X, Cen R, Xu FX, Xia J, Luo C, Cheng FL. Pathological analysis with new magnifying endoscopic classification of the gastric mucosal pattern. Zhongguo Neijing Zazhi. 2002;8:37-38. |
| 34. | Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H. pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848-854. [PubMed] |
| 35. | Chinese Society of Gastroenteroloy, Chinese Medical Association. Common opinions on chronic gastritis of China. Zhonghua Xiaohua Zazhi. 2000;20:199-201. |
| 36. | Liu YQ, Zhao H, Ning T, Ke Y, Li JY. Expression of 1A6 gene and its correlation with intestinal gastric carcinoma. World J Gastroenterol. 2003;9:238-241. [PubMed] |
| 37. | Zhou LY, Li JH, Lin SR, Jin Z, Ding SG, Huang XP, Xia ZW. Endoscopic diagnosis of intestinal metaplasia in gastric mucosa. Zhonghua Xiaohua Neijing Zazhi. 2001;18:84-86. |