Published online Nov 15, 2003. doi: 10.3748/wjg.v9.i11.2424
Revised: May 23, 2003
Accepted: June 2, 2003
Published online: November 15, 2003
AIM: To study the therapeutic mechanism of Ginkgo biloba exocarp polysaccharides (GBEP) on gastric cancer.
METHODS: Thirty patients with gastric cancer were treated with oral GBEP capsules. The area of tumors was measured by electron gastroscope before and after treatment, then the inhibitory and effective rates were calculated. The ultrastructures of tumor cells were examined by transmissional electron microscope. Cell culture, MTT, flow cytometry were performed to observe proliferation, apoptosis and changes of relevant gene expression of human gastric cancer SGC-7901 cells.
RESULTS: Compared with the statement before treatment, GBEP capsules could reduce the area of tumors, and the effective rate was 73.4%. Ultrastructural changes of the cells indicated that GBEP could induce apoptosis and differentiation in tumor cells of patients with gastric cancer. GBEP could inhibit the growth of human gastric cancer SGC-7901 cells following 24-72 h treatment in vitro at 10-320 mg/L, which was dose- and time-dependent. GBEP was able to elevate the apoptosis rate and expression of c-fos gene, but reduce the expression of c-myc and bcl-2 genes also in a dose-dependent manner.
CONCLUSION: The therapeutic mechanism of GBEP on human gastric cancer may relate to its effects on the expression of c-myc, bcl-2 and c-fos genes, which can inhibit proliferation and induce apoptosis and differentiation of tumor cells.
- Citation: Xu AH, Chen HS, Sun BC, Xiang XR, Chu YF, Zhai F, Jia LC. Therapeutic mechanism of ginkgo biloba exocarp polysaccharides on gastric cancer. World J Gastroenterol 2003; 9(11): 2424-2427
- URL: https://www.wjgnet.com/1007-9327/full/v9/i11/2424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i11.2424
Ginkgo biloba exocarp polysaccharide (GBEP) is polysaccharides isolated from ginkgo biloba exocarp. Many studies showed that GBEP was able to inhibit tumors and enhance immune function of tumor bearing mice. Clinical studies showed that GBEP had certain therapeutic effects but few toxic side-effects on patients with tumors, and had good prospects in clinical application[1-4]. This study was to investigate the therapeutic effect and mechanism of GBEP on human gastric cancer.
A total of 30 patients with gastric adenocarcinoma (20 males aged from 28 to 81 years old, 10 females aged from 52 to 75 years old) were all ascertained by the pathological examination in Jiangsu Provincial Subei People Hospital or the First People Hospital of Yangzhou, China. Their Karnofsky scores were all above 60. All the patients did not receive any other anti-tumor treatments recently.
Human gastric cancer cells (SGC-7901) purchased from Department of Cellular and Molecular Biology, Shanghai Institute of Biochemistry and Cell Biology Academia Sinica, were sub-cultured every 2 or 3 d.
GBEP was extracted from exocarp of ripe ginkgo biloba. The content of polysaccharides was higher than 80%. RPMI 1640 was from GibcoBRL (Maryland, USA). MTT and trypsin (1:250) were from Sigma (ST. Louis, USA). Monoclonal mouse anti-human c-myc and bcl-2 were purchased from Antibody Diagnostica Inc., USA. Monoclonal rabbit anti-human c-fos was from Santa Cruz (Santa Cruz, USA).
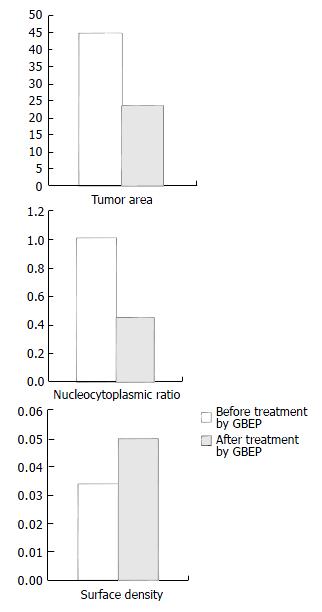
Influence of GBEP on gastric carcinoma patients GBEP capsules are composed of GBEP dry powder and a certain proportion of excipient, and 0.25 g per capsule. Each patient with gastric carcinoma was treated with oral GBEP capsule, 2 pills each time, twice a day, for over 30 d. Changes of tumor size were measured by electron gastroscope. The inhibitory rates (IR) were calculated according to the formula: IR = (tumor area before treatment - tumor area after treatment) - tumor area before treatment × 100%, which is used in the assessment of therapeutic effects. The assessment followed the clinical assessment standards for solid tumors made by WHO, which are classified as complete response (CR), partial response (PR), stable disease (SD) or no change (NC), and progressive disease (PD). The effective rate equals CR plus PR. At the meantime tumor biopsies were obtained for ultrastructural examination by transmissional electron microscope (HV-300). Images captured by transmissional electron microscope were analysed, and the nucleocytoplasmic ratio as well as the surface density of heterochromatin in the tumor cells were calculated before and after treatment.
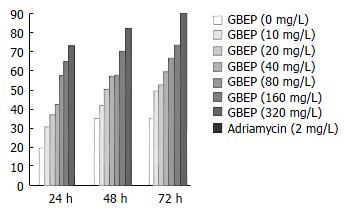
MTT experiment SGC-7901 cells growing exponentially were digested by 0.25% trypsin for 1-2 min, then washed in Hanks balanced salt solution (HBSS) for 2 times, and RPMI 1640 containing 10% new born bovine serum medium was added to adjust the cell density to 1 × 108 cells/L. After addition of the final cell suspensions of 100 μL/well, 96-well plates were put into an incubator containing 5% CO2, and incubated at 37 °C for 24 h. Then, 100 μL RPMI 1640 containing different concentrations of GBEP was added to each well. Each concentration had 3 wells, and the control was added with 100 μL RPMI 1640. They were cultured for 24 h, 48 h and/or 72 h. Fresh medium was changed per 24 h, and GBEP was added. Four hours before the end of culture, 10 μL MTT (the final concentration was 5 g/L) was added, and cultured for 4 h. Optical density (OD) values for each well were measured at 570 nm with the enzyme linked immunosorbent assay meter[5,6]. The inhibitory rates were calculated according to the formula: IR = [1 - (the mean of treated group)/(the mean of control group)] × 100%.
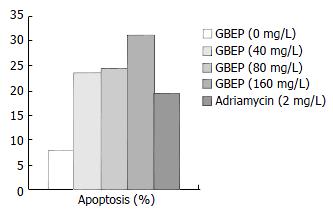
Measurement of cell apoptosis SGC-7901 cells growing exponentially were digested with 0.25% trypsin for 1-2 min. After that, the cells were washed 2 times with PBS buffer (pH7.2), counted and mixed with RPMI 1640 containing 10% new born bovine serum to create a final cell density of 2 × 108 cells/L. Four mL final cell suspension was added into each culture bottle, and cultured for 24 h in the condition of 5% CO2, at 37 °C. Then the culture bottles were randomly mixed with different concentrations of GBEP or a positive control drug adriamycin. The negative control group was mixed with an equal volume of RPMI 1640. Then, all the culture bottles were cultured for another 48 h. After that, they were digested and washed. At last they were fixed with alcohol and kept at 4 °C. The tumor cells were fixed with alcohol and kept in citrate buffer for at least 1 h after washed in PBS to create a final cell density of 1 × 109 cells/L, they were then centrifuged and mixed with 1800 μL solution A (trypsinization solution). After 10 min, they were mixed with 1500 μL solution B (RNASE) for 20 min, then mixed with 1500 μL solution C (PI) and filtered by a nylon net after 15 min. Finally, the apoptotic rate of the cells was examined.
Analysis of protein content Cell culture was carried out as previously described. The SGC-7901 cells were centrifuged (2000 ω/minute for 5 min) after washed in PBS, mixed with monoclonal mouse anti-human c-myc, or bcl-2, or rabbit anti-human c-fos, and kept at 4 °C for 45 min. The cells were washed in PBS and mixed with sheep anti-mouse or sheep anti-rabbit IgG and kept at 4 °C for another 45 min. After washed in PBS and centrifugation, the cells were mixed with 300 μL PBS in sediment and the rate of positive protein for c-myc, bcl-2 and c-fos gene was measured by flow cytometry.
Compared with that before treatment, the tumor area was apparently reduced, which was further proved by electron gastroscopy, and the inhibitory rate of GBEP on tumors was 53.5%. According to the standards proposed by WHO for the short-term therapeutic effectiveness of solid tumors, there were 2 cases of CR (6.7%), 20 PR (66.7%), 5 SD (16.7%), 3 PD (10%) in the 30 cases, and the total effective rate was 73.4%. Images captured by transmissional electron microscope showed that most of the cancer cells had sufficient euchromatins but deficient heterochromatins in the nuclei, the cancer cells had sufficient free ribosomes and deficient glycogens in the cytoplasm before treatment. After treatment with GBEP, most of the cancer cells had sufficient heterochromatins in the nuclei. Some cancer cells became pyknosis. Heterochromatin margination was seen in some of the cancer cells (in the course of apoptosis). Some euchromatins were dissolved, mitochondria were swollen, and rough endoplasmic reticulum was dilated. The results of image analysis showed that nucleocytoplasmic ratio in most of the cancer cells was reduced, surface density of heterochromatin was increased. Influence of GBEP capsules on the tumor area of gastric cancer, on the tumor cells’ nucleocytoplasmic ratio and the surface density are shown in Figure 1.
GBEP could inhibit SGC-7901 cell proliferation following 24-72 h treatment in vitro at 10-320 mg/L. Compared with the control group, the inhibition of SGC-7901 cell proliferation by GBEP was dose- and time-dependent (P < 0.01) (Figure 2).
DNA contents of human gastric cancer SGC-7901 cells were analysed by flow cytometry. The results showed that GBEP could induce apoptosis in SGC-7901 cells at a certain degree (Figure 3).
Protein contents of human gastric cancer SGC-7901 cells were analysed by flow cytometry. The results showed that GBEP could inhibit the expression of c-myc and bcl-2 genes, but enhance the expression of c-fos in SGC-7901 cells (Table 1).
| Group | Dose (mg/L) | Rate of positive protein sign (%) | ||
| c-myc | bcl-2 | c-fos | ||
| RPMI 1640 | - | 22.05 | 19.35 | 12.68 |
| GBEP | 40 | 20.33 | 15.29 | 17.35 |
| GBEP | 80 | 12.50 | 11.74 | 24.96 |
| GBEP | 160 | 7.34 | 7.17 | 45.26 |
| Adriamycin | 2 | 9.67 | 9.31 | 68.01 |
Gastric cancer is one of the most common malignant tumors in China. Surgical treatment is the main therapy of it. Anti-tumor drugs still play an important role in comprehensive therapy. Now cytotoxic compounds remain the main part of the chemotherapy drugs. The main defects of the cytotoxic compounds are the poor therapeutic effects on solid tumors, higher toxic side-effects and easy occurrence of drug resistance. Many Chinese drugs can enhance the immune function of the body. When used in the treatment, they showed less toxic side-effects but lower inhibitory rate on tumors.
Polysaccharides are big molecules linked by monosaccharides. The sugar-chain of polysaccharides can regulate cell proliferation, differentiation, growth and aging. They showed definite therapeutic effectiveness in anti-tumor therapy, and the ability to enhance body’s immune function, as well as a lower toxic side-effect[7-10]. For example, mushroom polysaccharides have already been used as a drug to regulate the organism reaction in clinical therapy and to prevent tumors in Japan. Umbellate pore fungus polysaccharides which were developed and used in clinical therapy in China, could reduce side-effects of chemotherapy and enhance the effects of chemotherapy against tumors.
We performed this clinical experiment by treating 30 gastric cancer patients with oral GBEP capsules. The images captured by electron gastroscope showed the average inhibitory rate of its capsules on gastric tumor was 53.5%. The effective rate was 73.4%. It indicated that GBEP had good clinical therapeutic effectiveness on gastric cancer.
Apoptosis is an active cellular process whereby individual cells are triggered to undergo self-destruction. Recent studies showed apoptosis played a main role in the prevention and treatment of tumors[11-13]. Anti-tumor effect of many chemotherapy drugs could induce apoptosis in tumor cells[14,15]. Cell apoptosis was regulated by genes[16,17]. We have known that apoptosis regulators can be divided into two kinds, namely apoptosis-inducing genes and apoptosis-inhibitory genes. Up-regulation of apoptosis-inducing gene expression could elevate the sensitivity of cells to factors or signals inducing apoptosis, and trigger apoptosis in this way. Up-regulation of apoptosis-inhibitory gene expression could reduce the sensitivity of cells to factors or signals inducing apoptosis, and apoptosis could be inhibited or delayed in this way. Bcl-2 was an important apoptosis-inhibitory gene[18-20], it included a nucleus molecule that can block cell apoptosis, prolong cell lives, accelerate DNA repairing, and thus promoting tumor genesis and development. So it could down-regulate cell apoptosis[21-25]. This clinical study and examination of cellular ultrastructures showed clues of apoptosis induced by GBEP in human gastric cancer cells. Using techniques of cell culture in vitro and flow cytometry, the contents of DNA and protein of human gastric cancer SGC-7901 cells were analysed. The results showed GBEP could increase SGC-7901 cell apoptosis rate, down-regulate bcl-2 at concentrations of 40-160 mg/L. It indicated that one of the therapeutic mechanisms of GBEP on gastric cancers might be that it induced tumor cell apoptosis. It also indicated that bcl-2 was involved in this process.
Malignant cells are similar to undifferentiated embryonic cells in morphology, function and metabolism. When tissue changes into malignancy, many phenotypes of the cells go back to the embryonic cell phenotypes, which is called de-differentiation or retro-differentiation. Malignant cells can be induced to differentiate towards normal cells in the presence of differentiation-inducer. Many malignant cells can approach to normal cells, even transform into normal cells completely, which is called re-differentiation or reversion. Change of cells from normal to malignancy is a break of the balance between proliferation and differentiation. Uncontrollable proliferation and de-differentiation are the characteristics of most malignant tumors. Differentiation-inducers can decelerate proliferation, enhance differentiation, thus creating a new normal balance. Like apoptosis, proliferation and differentiation are regulated by genes. C-myc is an important gene involved in the control of cell proliferation, and could up-regulate cell cycle progression, and induce cell proliferation[26-29]. C-fos gene is considered as an early response gene, and its expression level was in proportion to the differentiation degree of gastric cancer[30-32]. This clinical study and results of the cell ultrastructural examination showed clues of apoptosis induced by GBEP in human gastric cancer cells. The results of MTT experiment in vitro showed GBEP could inhibit the proliferation of human gastric cancer SGC-7901 cells. The results measured by flow cytometry showed GBEP could down-regulate the expression of c-myc gene and up-regulate the expression of c-fos in SGC-7901 cells at the concentrations of 40-160 mg/L. It indicates that inhibition on cell proliferation and inducement on cell differentiation might be involved in the therapeutic mechanism of GBEP on gastric cancer. C-myc and c-fos genes might contribute to the regulation of proliferation and differentiation.
We are very grateful to Drs. Huo-Ying Shi, Li-Ming Yuan and Wei-Dong Zhou, Center of Electroscope, Yangzhou University, and Mei-Zao Le, Department of Pathology, Bayi Hospital, Nanjing, China for their technical assistance in ultrastructural analysis; Drs. Zhi-Jiang Wu, L-Rong Men, Department of Cellular and Molecular Biology, Shanghai Institute of Biochemistry and Cell Biology, Academica Sinica for their technical assistance in flow cytometry.
Edited by Zhang JZ and Wang XL
| 1. | Xu AH, Chen HS, Xiang XR, Gu WR, Zhang HQ. The suppressive effects of ginkgo biloba exocarp polysaccharides (GBEP) on tumor in mice. Zhongyao Iaoli yu Linchuang. 1996;12:24-26. |
| 2. | Chen HS, Xu AH, Wang Y, Wang Q, Wang XL. Influence of ginkgo biloba exocarp polysaccharides (GBEP) on interleukin-2 (IL-2) activity and solubility interleukin-2 receptor (sIL-2R) level in mice under lower immune function. Zhongyao Yaoli yu Linchuang. 2001;17:17-19. |
| 3. | Zhai F, Chen HS. Clinical observation on effects of ginkgo biloba exocarp polysaccharides (GBEP) in Treatment of 84 Cases in cancer of middle-late stage. Liaoning Zhongyi Zazhi. 2002;29:564. |
| 4. | Xu A, Chen H, Wang L, Wang Q. [Influence of Ginkgo biloba L. exocarp polysaccharides on serum superoxide dismutase activity and malondialdehyde level in mice under different states]. Zhongguo Zhongyao Zazhi. 1998;23:746-747, back cover. [PubMed] |
| 5. | Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J immunol Methods. 1983;65:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38285] [Cited by in RCA: 39395] [Article Influence: 938.0] [Reference Citation Analysis (0)] |
| 6. | Wu Q, Chen Z, Su W. Mechanism of inhibition on activator protein-1 activity by all-trans retinoic acid in gastric cancer cells. Chin Med J (. Engl). 2000;113:972-976. [PubMed] |
| 7. | Liu C, Gao P, Qian J, Yan W. [Immunological study on the antitumor effects of fungus polysaccharides compounds]. Wei Sheng Yan Jiu. 2000;29:178-180. [PubMed] |
| 8. | Wang Z, Wang Y, Huang Z, Zhong S, Wu Y, Yu L. [Study on antitumor effect and mechanism of aloe polysaccharides]. Zhong Yao Cai. 2001;24:350-353. [PubMed] |
| 9. | Kodama N, Komuta K, Sakai N, Nanba H. Effects of D-Fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol Pharm Bull. 2002;25:1647-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Lu X, Su M, Li Y, Zeng L, Liu X, Li J, Zheng B, Wang S. Effect of Acanthopanax giraldii Harms Var. Hispidus Hoo polysaccharides on the human gastric cancer cell line SGC-7901 and its possible mechanism. Chin Med J (. Engl). 2002;115:716-721. [PubMed] |
| 11. | Yan J, Xu YH. Tributyrin inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2003;9:660-664. [PubMed] |
| 12. | Su M, Dai M, Lü X, Li H, Liu J. [Effect of traditional Chinese medicine compounds Aining on the expression of apoptosis inducing genes of human gastric cancer cell]. Zhong Yao Cai. 2002;25:563-566. [PubMed] |
| 13. | Gao F, Yi J, Shi GY, Li H, Shi XG, Tang XM. The sensitivity of digestive tract tumor cells to As2O3 is associated with the inherent cellular level of reactive oxygen species. World J Gastroenterol. 2002;8:36-39. [PubMed] |
| 14. | Kimura H, Konishi K, Kaji M, Maeda K, Yabushita K, Tsuji M, Ogino H, Satomura Y, Unoura M, Miwa A. Apoptosis, cell proliferation and expression of oncogenes in gastric carcinomas induced by preoperative administration of 5-fluorouracil. Oncol Rep. 2000;7:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Sugamura K, Makino M, Shirai H, Kimura O, Maeta M, Itoh H, Kaibara N. Enhanced induction of apoptosis of human gastric carcinoma cells after preoperative treatment with 5-fluorouracil. Cancer. 1997;79:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Masutani M, Suzuki J, Matsuda T, Dochin A, Sadaoka K, Nomura A, Ohira K, Takahashi K, Yamazaki K, Dosaka-Akita H. Increased apoptosis associated with depressed type of early intestinal gastric cancer. Jpn J Cancer Res. 2001;92:1214-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. [PubMed] |
| 18. | Cao J, Qiao Y, Min J. [Bcl-2 anti-sense oligonucleotide sensitizes Fas-mediated apoptosis of gastric cancer cells]. Zhonghua Zhongliu Zazhi. 2000;22:466-468. [PubMed] |
| 19. | Zhou HB, Zhu JR. Paclitaxel induces apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2003;9:442-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796-800. [PubMed] |
| 21. | Höfler H, Becker KF. Molecular mechanisms of carcinogenesis in gastric cancer. Recent Results Cancer Res. 2003;162:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Dixon D, Flake GP, Moore AB, He H, Haseman JK, Risinger JI, Lancaster JM, Berchuck A, Barrett JC, Robboy SJ. Cell proliferation and apoptosis in human uterine leiomyomas and myometria. Virchows Arch. 2002;441:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Wang NS, Unkila MT, Reineks EZ, Distelhorst CW. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem. 2001;276:44117-44128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Li JQ, Chen RC, Cai KX, Ye ZY. [Apoptosis of human gastric cancer cell induced by photochemical riboflavin]. Ai Zheng. 2003;22:253-256. [PubMed] |
| 25. | Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, Su WJ. Induction of apoptosis by TPA and VP-16 is through translocation of TR3. World J Gastroenterol. 2002;8:446-450. [PubMed] |
| 26. | Chen JP, Lin C, Xu CP, Zhang XY, Fu M, Deng YP, Wei Y, Wu M. Molecular therapy with recombinant antisense c-myc adenovirus for human gastric carcinoma cells in vitro and in vivo. J Gastroenterol Hepatol. 2001;16:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Liu H, Lo CR, Jones BE, Pradhan Z, Srinivasan A, Valentino KL, Stockert RJ, Czaja MJ. Inhibition of c-Myc expression sensitizes hepatocytes to tumor necrosis factor-induced apoptosis and necrosis. J Biol Chem. 2000;275:40155-40162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Zhu GH, Wong BC, Eggo MC, Ching CK, Yuen ST, Chan EY, Lai KC, Lam SK. Non-steroidal anti-inflammatory drug-induced apoptosis in gastric cancer cells is blocked by protein kinase C activation through inhibition of c-myc. Br J Cancer. 1999;79:393-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Ishii HH, Gobé GC, Pan W, Yoneyama J, Ebihara Y. Apoptosis and cell proliferation in the development of gastric carcinomas: Associations with c-myc and p53 protein expression. J Gastroenterol Hepatol. 2002;17:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Tsuji M, Funahashi S, Takigawa M, Seiki M, Fujii K, Yoshida T. Expression of c-fos gene inhibits proteoglycan synthesis in transfected chondrocyte. FEBS Lett. 1996;381:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Ito N, Hirose M, Takahashi S. Cell proliferation and forestomach carcinogenesis. Environ Health Perspect. 1993;101 Suppl 5:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Kane S, Prentice MA, Mariano JM, Cuttitta F, Jakowlew SB. Differential induction of early response genes by adrenomedullin and transforming growth factor-beta1 in human lung cancer cells. Anticancer Res. 2002;22:1433-1444. [PubMed] |