Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2350
Revised: February 6, 2003
Accepted: February 13, 2003
Published online: October 15, 2003
AIM: To explore a safe, efficient, and cost-effective technique for local thermo-ablation of hepatic tumors.
METHODS: The livers of 16 healthy rabbits were thermocoagulated by diode-laser with a hand-made scanner fiber tip, 6 w for 10 min. At the same time, the temperature was measured at 5 and 10 mm from the laser tip. Liver function 7 d post-thermocoagulation was compared to pre-thermocoagulation. Pathological changes were also studied 1 month after laser thermocoagulation.
RESULTS: All the rabbits lived and the temperature of hepatic tissues at 0 mm, 5 mm, 10 mm from laser tip reached 96.39 ± 3.97 °C, 60.79 ± 6.21 °C, 46.10 ± 4.58 °C, respectively after 10 min thermocoagulation. There was no significant change in liver function. The hepatic thermocoagulated necrosis and the surrounding fibrosis was 26.0 mm in diameter. Light microscopy observation revealed no surviving cells in the coagulated area.
CONCLUSION: Hepatic tissue can be locally ablated safely and effectively by diode-laser with scanner fiber tip, and this technique may be a new method to treat hepatic tumors.
- Citation: Hong DF, Peng SY, Li SY, Tong LM. Experimental study of diode-laser induced thermocoagulation on hepatic tissue with scanner fiber tip. World J Gastroenterol 2003; 9(10): 2350-2352
- URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2350.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2350
In recent years, thermo-ablation of liver cancer has received increasing attention. Generally this can be achieved by radio-frequency (RF) or Nd:YAG laser. Several factors have prevented these techniques from gaining widespread use including availability and cost, in addition to some uncertainty about their therapeutic effectiveness. With these concerns in mind, it is appropriate to consider a new technique for thermo-ablation that we believe is safe, stable, convenient and cost effective. Semiconductor taper-scattered light for thermo-ablation of liver tissue in an experimental model is advanced as such a technique with details presented as follows.
Hand-made semiconductor taper-scattered laser therapeutic system (HTLTS) has a wavelength of 810 ± 10 nm. The working tip is 10 mm long and 1 mm in diameter. Power utilization was 6 w and after dispersion output power was at 2.1 w with an exposure time of 10 min. Sixteen adult rabbits were obtained from the Animal Laboratory of Zhejiang University, weighing 2 to 3 kg.
Intravenous anesthesia was administered via ear vein using 2% sodium pentothal. The abdomen was entered with a midline incision and right or left lobes of the liver were exposed. A 18G PTC needle was introduced into the liver and through it, the laser tip was advanced into the liver tissue before turning on the power for the laser. Care was taken to advance the laser tip fully into the liver tissue. Power input was 6 w and 2.1 w after dispersion.
For temperature measurement during laser application, a nickel-chrome-nickel thermocouple 300 μm in diameter was placed intrahepatically at 5 and 10 mm from the laser tip. Temperature was continuously measured during application. After thermocoagulation the abdomen was closed with silk suture. Liver function was measured before surgery and 7 d afterwards. One month after irradiation the rabbits were sacrificed and the liver specimen was fixed in formaldehyde. Multiple sections were taken from the ablative and surrounding area in the verticle plane of the optical fiber. After H&E staining the sections were examined grossly and microscopically with particular attention to the diameter of the coagulated area. Liver function data were analyzed using the t-test with the “SAS” software.
The temperature variation is shown in Table 1. Note that two of the rabbits were irradiated for only six minutes because of carbonization of tissue at the center of the optical fiber.
| Time (Seconds) | Temperature (°C) | ||
| 0 mm | 5 mm | 10 mm | |
| 0 | 34.00 ± 0.00 | 34.00 ± 0.00 | 34.00 ± 0.00 |
| 120 | 55.70 ± 4.81 | 40.50 ± 1.47 | |
| 240 | 58.75 ± 5.65 | 43.30 ± 2.54 | |
| 360 | 60.26 ± 5.52 | 45.00 ± 2.67 | |
| 480 | 59.89 ± 6.65 | 46.10 ± 3.87 | |
| 600 | 96.39 ± 3.97 | 60.79 ± 6.21 | 46.10 ± 4.58 |
Liver function changes are shown in Table 2.
| Pre-coagulation | 7 d after coagulation | P | |
| ALT (IU/L) | 67.78 ± 24.98 | 77.11 ± 49.79 | > 0.05 |
| AST (IU/L) | 87.22 ± 58.82 | 66.55 ± 44.75 | > 0.05 |
| TBIL (mg/dL) | 1.84 ± 1.79 | 1.21 ± 0.51 | > 0.05 |
| ALB (g/L) | 17.90 ± 3.98 | 14.77 ± 2.79 | > 0.05 |
| TP (g/L) | 66.54 ± 13.95 | 59.40 ± 9.55 | > 0.05 |
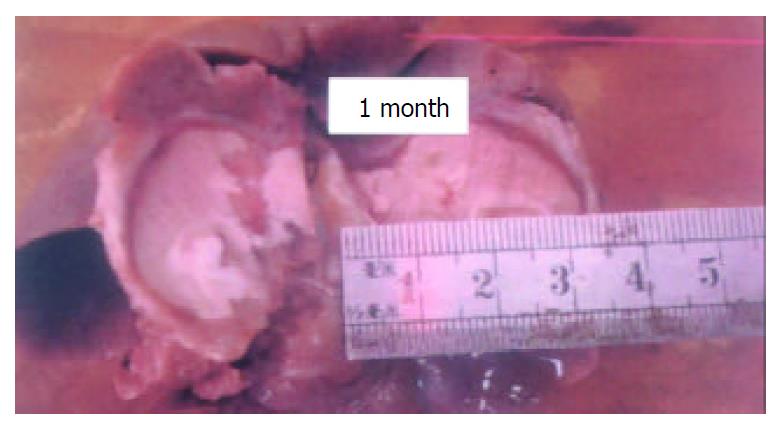
All rabbits were alive after the procedure. When the animals were sacrificed after one month the livers were cut in the vertical plane of the optical fiber and the gray coagulated necrotic tissue was measured. Gross evaluation demonstrated a diameter of 23 mm with a perimeter zone of 3 mm (Figure 1). Under light microscopy the central zone of coagulation necrosis was followed by a layer showing inflammatory cell infiltration and finally the layer of fibrosis, the last two were 0.5 mm and 2.4 mm, respectively.
Currently the optimal treatment for liver cancer is surgical excision with curative intent. However, because of the frequency of coexistent cirrhosis and the difficulty of early detection, only 25 to 30 percent of newly diagnosed patients with primary or metastatic liver cancer undergo potential curative resection and the recurrence rate is at least 50%[1-2]. This means many patients are not even candidates for a surgical procedure and have little chance even for palliation, which has led to increasing interest in alternatives such as thermo-ablative procedures including radio-frequency, Nd:YAG laser and microwave. However, limited benefit, high cost and difficulties in technical application have limited the usefulness of these modalities as well[3-6].
The basis for laser thermo-ablation is that light energy can be absorbed by tissues after its conversion into heat, resulting in deep penetration with therapeutic destruction of tumor tissues. The temperature and therefore the extent of destruction are controlled by the intensity and duration of the energy transmitted. This has already been shown effective using Nd:YAG laser as the energy source. However high cost and difficulties in use have restricted its application in this setting[6-9]. More recently, semi-conductor focused laser energy has come to the forefront due to decreased cost, stable-performance, durability and ease of use. In addition it does not require water cooling equipment[7,8]. In this report, we have examined a similar process using semiconductor taper-scattered light. We have shown 1064 nm Nd:YAG and 800-900 nm semiconductor laser light both exhibit deep penetration in tissue. Dong[10] has shown normal liver cells necrotize in one minute with a temperature of 54 °C and immediately with a temperature of 60 °C. In addition he has shown liver carcinoma cells are more sensitive than normal cells if the temperature is less than 130 °C. We also know penetration of laser energy is significantly reduced by vaporization and carbonization of tissues. Obviously, to enlarge the treatment area and avoid injury to adjacent areas it is necessary to elevate the temperature of the treatment area but not so high as to cause vaporization and carbonization. It is our opinion that semiconductor taper-scattered laser light may be superior to other systems in this regard. HTLTS can convert high density linear laser energy into up-tip taper scattered laser light which is lower in energy and density as shown in Figure 2 and Figure 3. In effect it can increase the power of laser light equipment, enlarge the treatment area, shorten the duration of application and avoid injury to important areas adjacent to the treatment area such as major hepatic vessels. Specifically, the power is only 6 watts and after scatter only 2.1 W. Ten minutes after thermocoagulation the temperature at the center of the optical fiber is 96.39 ± 3.97 °C and 10 mm from the center the temperature is 46.1 ± 4.58 °C. One month after the procedure the diameter of necrosis was 26 mm and there was no viable tissue in this area. HTLTS is more efficient than Nd:YAG or semiconductor focused laser light. In the study by Ping Liang[5] Nd:YAG provided only 15 mm necrosis using 2 to 6 W energy after 15 to 40 min application. Fan[7] demonstrated only 7-13 mm necrosis with semiconductor focused laser energy from a British laser using 2 W and 2800-8400 J respectively. Injury beyond the intended area is less likely since it is not necessary to place the tip of the instrument exactly in the center of the lesion and this makes the procedure also applicable for more superficial lesions. The optical fiber can be separated from the tissue with the catheter thus avoiding contamination from the blood and the length of the fiber tip can be altered to fit the diameter of the tumor. Furthermore, the optical fiber can be sterilized and reused, the fiber is flexible, allowing access to areas one side or the other of the primary objective. Overall, HTLTS is more convenient, economical and effective than the currently available ablative procedures.
In regards to the effect of HTLTS on hepatic carcinoma or metastatic lesions, we have shown greater penetration than in normal tissue. For example, the penetration of YAG (wavelength 670 nm) in normal liver tissue, liver sclerosis, HCC, and metastatic lesions is 0.75 mm, 0.97 mm, 1.43 mm and 2.78 mm, respectively[11]. HCC is also more sensitive to thermal ablation than normal tissue. Again HTLTS is more effective and efficient than the other methods. HTLTS can also be applied to the clinical area easily. The fiber is passed into the intended tissue through a 18G paracentesis needle guided by B-ultrasound. The length of the fiber tip can be adjusted according to the size of the target tissue. Special techniques are also available to utilize more than one fiber at a time to improve the resulting effect according to the exact size and location of the target tissue.
In conclusion, we believe that the foregoing report indicates HTLTS may be the best technique yet devised for thermo-ablation of hepatic lesions.
Edited by Pang LH
| 1. | Cance WG, Stwart AK, Menck HR. The national cancer data base report on treatment patterns for hepatocellular carcinoma: im-proved of surgically resected patiens, 1985-1986. Cancer. 2000;88:912-920. |
| 2. | Wu MC, Chen H, Sheng F. Surgical treatment of primary liver cancer: report of 5524 cases. Zhonghua Waike Zazhi. 2001;39:25-28. |
| 3. | Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 805] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 4. | Ohmoto K, Miyake I, Tsuduki M, Shibata N, Takesue M, Kunieda T, Ohno S, Kuboki M, Yamamoto S. Percutaneous Microwave coagulation therapy for unresectable hepatocellular carcinoma. Hepatogastroenterology. 1999;46:2894-2900. |
| 5. | Liang P, Dong BW, Gu Y, Li JH, Yu XN, Su L, Zhang Y. Effect of Nd: YAG laser coagulation on hepatic tissue and its application to hepatic cancer. Zhonghua Liliao Zazhi. 1999;22:158-160. |
| 6. | Heisterkamp J, van Hillegersberg R, Ijzermans JN. Interstitial laser coagulation for hepatic tumours. Br J Surg. 1999;86:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Fan XH, Shen TZ, Lu W, Gong DX. Laser ablation of liver malig-nant tumor. Zhongguo Yixue Jishuanji Chengxiang Zazhi. 2000;6:404-407. |
| 8. | Devaux BC, Roux FX, Nataf F, Turak B, Cioloca C. High-power diode laser in neurosurgery: clinical experience in 30 cases. Surg Neurol. 1998;50:33-39; discussion 39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Zhang ZX, Jiang DZ. Laser-Tissue Interactions: Foundaments and Application. 1st ed. Xi'an: Xian Jiaotong University Publishing House 1999; 64-65. |
| 10. | Dong BW, Liang P, Yu XL, Zeng XQ, Wang PJ, Su L, Wang XD, Xin H, Li S. Sonographically guided microwave coagulation treatment of liver cancer: an experimental and clinical study. AJR Am J Roentgenol. 1998;171:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Li DJ, Hu ZS. Tumor thermotherapy. 1st ed. Henan: Henan Medi-cal University Publishing House 1995; 186-187. |