Published online Oct 15, 2002. doi: 10.3748/wjg.v8.i5.918
Revised: June 4, 2002
Accepted: June 8, 2002
Published online: October 15, 2002
AIM: Toosendanin is a pre-synaptic blocker at the neuromuscular junction and its inhibitory effect is divided into an initial facilitative/stimulatory phase followed by a prolonged inhibitory phase. The present study investigated whether the subsequent inhibitory phase was due to exhaustion of the secretory machinery as a result of extensive stimulation during the initial facilitative phase. More specifically, this paper examined whether toosendanin could directly inhibit the secretory machinery in exocrine cells.
METHODS: Rat pancreatic acinar cells were isolated by collagenase digestion. Secretion was assessed by measuring the amount of amylase released into the extracellular medium as a percentage of the total present in the cells before stimulation. Cholecystokinin (CCK)-induced increases in intracellular calcium in single cells were measured with fura-2 microfluorometry.
RESULTS: Effects of toosendanin on CCK-induced amylase secretion and calcium oscillations were investigated. Toosendanin of 87-870 μM had no effect on 10 pM-100 nM CCK-stimulated amylase secretion, nor did 8.7-870 μM toosendanin inhibit 5 pM CCK-induced calcium oscillations. In contrast, 10 nM CCK1 receptor antagonist FK 480 completely blocked 5 pM CCK-induced calcium oscillations.
CONCLUSION: The pre-synaptic "blocker" toosendanin is a selective activator of the voltage-dependent calcium channels, but does not interfere with the secretory machinery itself.
- Citation: Cui ZJ, He XH. The pre-synaptic blocker toosendanin does not inhibit secretion in exocrine cells. World J Gastroenterol 2002; 8(5): 918-922
- URL: https://www.wjgnet.com/1007-9327/full/v8/i5/918.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i5.918
Toosendanin is a tetracyclic triterpenoid isolated from the seeds and barks of Melia toosendan Seib. et Zucc and Melia azedarach L. It has been used as an anthelmintic for many centuries, and has also been found to have pesticidal effects[1-5], and have anti-botulismic and other effects in whole animals[6,7]. Work on nerve-muscle and other preparations (neuromuscular junction)[8-14] has established that toosendanin is a potent, long-lasting pre-synaptic inhibitor. The neuromuscular blocking effect of toosendanin is divided into two phases: an early stimulatory phase that is due to direct activation of voltage-dependent calcium channels[8,12-18], and a delayed inhibition[9,14]. However, it is not known whether or not the delayed blockade of the neuromuscular transmission is due to direct interference with the secretory machinery involved in neurotransmitter release.
Whether toosendanin has any direct effect on the secretory machinery can only be examined in secretory cells with no voltage-dependent calcium channels. If indeed the delayed blocking effect of toosendanin is due to direct inhibition of the secretory machinery, only will an inhibitory phase be observed in secretory cells which lack voltage-dependent calcium channels. The pancreatic acinar cell is an ideal model in which to address this question. The molecular mechanisms of secretion in pancreatic acinar cells are well elucidated[19-24], and these non-excitable cells have no voltage-dependent calcium channels[25,26]. Therefore, this study examined if toosendanin has any inhibitory effect on secretion induced by a physiological secretagogue, cholecystokinin (CCK), in freshly isolated rat pancreatic acinar cells.
CCK octapeptide, α-amylase and amylose azure were purchased from Sigma (St. Louis, MO, USA), Cell-Tak was purchased from Collaborative Biomedicals (Bedford, MA, USA). Toosendanin was a gift from Professor He LI (Department of Chemistry, Beijing Normal University). Collagenase P was bought from Boehringer Mannheim (Mannheim, Germany). Fura-2 AM was purchased from Molecular Probes (Eugene, OR, USA). (s)-N-[1- (2-fluorophenyl)-3,4,6,7-tetrahydro-4-oxo-pyrrolo[3,2,1-jk][1,4]benzodiazepin-3yl]-1H-indole-2-carboximide (FK480) was donated by Fujisawa Pharmaceutical Co. Ltd. (Osaka, Japan). Toosendanin and FK480 were both dissolved in DMSO as stock solutions before dilution to final concentration.
Pancreatic acini were isolated from male Sprague-Dawley rats with body weight ranged from 170 g to 250 g according to the method reported previously[23,27,28].
Isolated acini were aliquoted into 2 mL portions and stimulated at 37 °C in a shaking water bath (50 cycle·min-1) for 30 min. The amylase secreted into the buffer was assayed according to the procedures reported previously[29-32] and expressed as percentage of the total present in the acini before stimulation.
Ten microlitre of 1 mM Fura-2 AM was added to1 mL of isolated acini (final concentration 10 μM) and the mixture was incubated in a shaking water bath at 37 °C and 50 cycle·min-1 for 40 min. Fura-2-loaded acini were attached to the cover-slip of a Sykus-Moore chamber and perfused on the stage of an Olympus fluorescence miscroscope (IX70) attached to a microfluorometric calcium measurement system (M40, Photon Technology International, NJ, USA). Calcium increases were expressed as fluorescence ratios measured at 510 nm (F340/F380)[23,27,33-35].
Standard buffer used in this work was composed of (all in mM) NaCl 118, KCl 4.7, MgCl2 1.16, CaCl2 2.5, NaH2PO4 1.16, glucose 5.6, bovine serum albumin 2 mg·mL1, soybean trypsin inhibitor 0.1 mg·mL-1, N-(2-hydroxyethyl) piperazine-N'(2-ethanesulfonic acid) (HEPES) 10, MEM amino acid mixture (GIBCOBRL, Grand Island, NY, USA) 2% and glutamine 2. The buffer was adjusted for pH to 7.4 with 4 mM NaOH and oxygenated with O2 for 30 min before use.
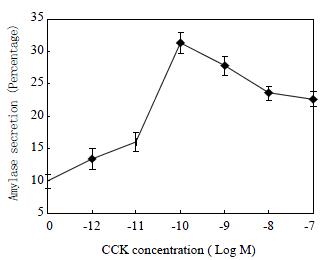
CCK stimulated amylase secretion from the freshly isolated rat pancreatic acini in a concentration-dependent manner (Figure 1). The maximum stimulation was achieved at CCK concentration of 100 pM, with a percentage secretion of 33 ± 1.6 (n = 6). This bell-shaped dose response curve is consistent with previous report[36].
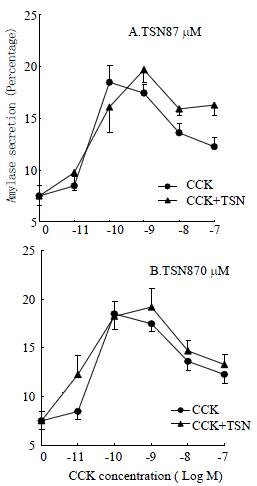
In separate experiments, acini were first incubated with toosendanin at 870 μM or 87 μM for 10 min before stimulation with CCK for a further 30 min in the continued presence of toosendanin (Figure 2). When toosendanin was added at 87 μM, the maximum stimulating CCK concentration shifted from 100 pM (18.2 ± 1.6, n = 6) to 1 nM (19.7 ± 1.2, n = 6). With the addition of toosendanin at 870 μM, the maximum CCK concentration also shifted from 100 pM (18.2 ± 1.6, n = 6) to 1 nM (19.2 ± 1.9, n = 4). The slight rightward shift indicates a mild but statistically insignificance inhibition (Student's t test, P > 0.05). In control experiments, neither 0.01% solvent DMSO nor toosendanin at each concentration used had any effect on amylase secretion (data not shown).
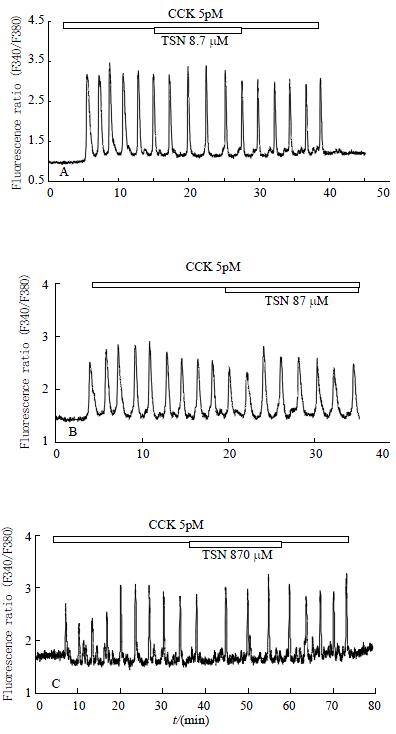
CCK of 5 pM induced regular calcium oscillations in perfused rat pancreatic acinar cells (Figure 3), which is consistent with previous reports[27,28]. Addition of toosendanin at 8.7 μM to the CCK-stimulated acinar cells for 10-20 min had no apparent effect on CCK-induced calcium oscillations (Figure 3A, n = 4). Even when toosendanin was increased to 87 μM, there was still no obvious inhibition observed (Figure 3B, n = 7). At 870 μM, toosendanin induced a very mild inhibition: a single spike appeared missing in the trace shown (Figure 3C, n = 8).
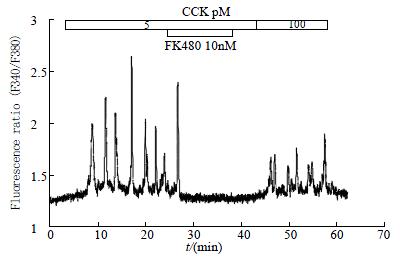
For comparison, FK480, an antagonist for CCK1 receptors[37-39], produced immediate and complete inhibition of 5 pM CCK-induced calcium oscillations (Figure 4, n = 5). FK480 at 10 nM abolished 5 pM CCK-induced calcium oscillations immediately upon addition, reducing the calcium to pre-stimulation level (Figure 4). After washout of FK480, calcium oscillations did not re-appear immediately, indicating that FK480 might bind to the acinar cells very tightly. It was possible, however, to re-introduce calcium oscillations when CCK was increased to 100 pM. At a lower FK480 concentration of 1 nM, a much longer time was needed before complete abolition of 5 pM CCK-induced Ca2+ oscillations was observed (data not shown).
The present work demonstrated that toosendanin, a pre-synaptic inhibitor of the neuromuscular transmission[7,9,13,14], had little effect on CCK-stimulated amylase secretion. Although treatment with toosendanin at both concentrations tested (87 μM, 870 μM) resulted in a rightward shift in concentration of CCK that was needed to induce a maximum stimulation, i.e. from 100 pM in untreated cells to 1 nM in treated cells (Figure 2)[36], the toosendanin inhibition was not statistically significant for each CCK concentration (P > 0.05).
Toosendanin of 8.7-87 μM had no effect on 5 pM CCK-induced calcium oscillations. At a much higher concentration of 870 μM, a mild inhibition was observed because one spike seemed missing (Figure 4). This lack of marked inhibition of toosendanin on CCK-induced calcium oscillations is in sharp contrast with the complete blockade of CCK-induced calcium oscillations by FK480, a CCK1 receptor antagonist[38,40,41]. Toosendanin at the neuromuscular junction had dual effects, a fast-onset stimulation which lasts about 40 min followed by a long-lasting inhibition[7-9,12-14]. The fast phase has been postulated to be due to activation of voltage-dependent calcium channels[8,12-18]. However, toosendanin had no effect on CCK-induced calcium oscillations in rat pancreatic acinar cells. This indicates that toosendanin had no effect on stores-operated calcium channels as they are the only calcium channels existing in the freshly isolated pancreatic acinar cells[20,26] and are important for the continued presence of calcium oscillations[23,27].
In view of the above findings, the pre-synaptic blocking effect of toosendanin should be looked at under a new light. The delayed inhibition of neuromuscular transmission could just be due to massive stimulation of synaptic vesicle fusion after activation of the voltage-dependent calcium channels at the nerve terminal, resulting in the depletion of synaptic vesicles and delayed depression. The fact that toosendanin administration in rat leads to a decrease in synaptic vesicles[42,43] strongly supports this hypothesis. The initial strong stimulation of voltage-dependent calcium channels would afford the early stimulatory effects of toosendanin, providing an antidote to botulism[6,18].
An activator for voltage-dependent calcium channels as toosendanin may be, a long-lasting inhibition of the neuromuscular junction[7-9,12-14] or an extended period of synaptic vesicle depletion would require the stimulatory effect of toosendanin to be long-lasting. This stimulatory effect has indeed been found to last 40 min in nerve-muscle preparations[7-9,12-14]. It is well known that voltage-dependent calcium channels inactivate rather quickly after opening, although with different kinetics[44-49]. Therefore, the long-lasting effect must be due to something other than constant opening of the calcium channels. It is known that intracellular calcium signals are subsequently encoded into activation of calcium/calmodulin-dependent protein kinases[50-52], and short-duration calcium signals could be transformed into long-lasting activation of calcium/calmodulin-dependent protein kinase II[53], therefore it would be interesting in the future to further investigate the possible effect of toosendanin on calcium oscillations in excitable cells, and on oscillation-associated activation of calcium/calmodulin-dependent protein kinase II. It would also be interesting to identify which types of voltage-dependent calcium channels are activated by toosendanin since a number of them are involved in neurotransmitter release[54-56]. In this conjunction, it is important to note that decreased calcium influx into motor nerve terminals has been found to recruit additional neuromuscular junctions during the synapse elimination period[57].
Edited by Liu HX
| 1. | Wang WL, Wang Y, Chiu S. The toxic chemical factors in the fruits of Melia Azadarach and their bioactivities toward Pieris rapae. Kunchong Xuebao. 1994;37:20-24. |
| 2. | Céspedes CL, Alarcón J, Aranda E, Becerra J, Silva M. Insect growth regulator and insecticidal activity of beta-dihydroagarofurans from Maytenus spp. (Celastraceae). Z Naturforsch C. 2001;56:603-613. [PubMed] |
| 3. | Céspedes CL, Martínez-Vázquez M, Calderón JS, Salazar JR, Aranda E. Insect growth regulatory activity of some extracts and compounds from Parthenium argentatum on fall armyworm Spodoptera frugiperda. Z Naturforsch C. 2001;56:95-105. [PubMed] |
| 4. | Céspedes CL, Calderón JS, Lina L, Aranda E. Growth inhibitory effects on fall armyworm Spodoptera frugiperda of some limonoids isolated from Cedrela spp. (Meliaceae). J Agric Food Chem. 2000;48:1903-1908. [PubMed] |
| 5. | Tada K, Takido M, Kitanaka S. Limonoids from fruit of Melia toosendan and their cytotoxic activity. Phytochemistry. 1999;51:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Zou J, Miao WY, Ding FH, Meng JY, Ye HJ, Jia GR, He XY, Sun GZ, Li PZ. The effect of toosendanin on monkey botulism. J Tradit Chin Med. 1985;5:29-30. [PubMed] |
| 7. | Zhao WQ, Feng H, Bennett P, Ng KT. Inhibition of intermediate-term memory following passive avoidance training in neonate chicks by a presynaptic cholinergic blocker. Neurobiol Learn Mem. 1997;67:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Ding J, Xu TH, Shi YL. Different effects of toosendanin on perineurially recorded Ca (2+) currents in mouse and frog motor nerve terminals. Neurosci Res. 2001;41:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Wang ZF, Shi YL. Modulation of inward rectifier potassium channel by toosendanin, a presynaptic blocker. Neurosci Res. 2001;40:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Wang ZF, Shi YL. Inhibition of large-conductance Ca (2+)-activated K (+) channels in hippocampal neurons by toosendanin. Neuroscience. 2001;104:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Wang ZF, Shi YL. Toosendanin-induced inhibition of small-conductance calcium-activated potassium channels in CA1 pyramidal neurons of rat hippocampus. Neurosci Lett. 2001;303:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Hua-Yu H, Cheng-Wen Z, Yu-Liang S. Toosendanin facilitates [3H]noradrenaline release from rat hippocampal slices. Nat Toxins. 1996;4:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Xu Y, Shi Y. Action of toosendanin on the membrane current of mouse motor nerve terminals. Brain Res. 1993;631:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Shih YL. Abolishment of non-quantal release of acetylcholine from the mouse phrenic nerve endings by toosendanin. Jpn J Physiol. 1986;36:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Shi YL, Furuya K, Wang WP, Terekawa S, Xu K, Yamagishi S. Calcium conductance increase by toosendanin in NG108-15 cells. Kexue Tongbao. 1993;38:836-839. |
| 16. | Ye Q, Qu AL, Zhang CG, Xu T, Zhou Z. Effects of toosendanin on the [Ca2]i on rat chromaffin cells. Zhongguo Shenjing Kexue Zazhi. 2001;17:105-108. |
| 17. | Shi YL, Chen WY. Effect of Toosendanin on acetylcholine level of rat brain, a microdialysis study. Brain Res. 1999;850:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Shih YL, Hsu K. Anti-botulismic effect of toosendanin and its facilitatory action on miniature end-plate potentials. Jpn J Physiol. 1983;33:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Schulz I, Krause E, González A, Göbel A, Sternfeld L, Schmid A. Agonist-stimulated pathways of calcium signaling in pancreatic acinar cells. Biol Chem. 1999;380:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Camello C, Pariente JA, Salido GM, Camello PJ. Sequential activation of different Ca2+ entry pathways upon cholinergic stimulation in mouse pancreatic acinar cells. J Physiol. 1999;516:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Cancela JM, Van Coppenolle F, Galione A, Tepikin AV, Petersen OH. Transformation of local Ca2+ spikes to global Ca2+ transients: the combinatorial roles of multiple Ca2+ releasing messengers. EMBO J. 2002;21:909-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Leite MF, Burgstahler AD, Nathanson MH. Ca2+ waves require sequential activation of inositol trisphosphate receptors and ryanodine receptors in pancreatic acini. Gastroenterology. 2002;122:415-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Cui ZJ, Kanno T. Cholecystokinin analog JMV-180-induced intracellular calcium oscillations are mediated by inositol 1,4,5-trisphosphate in rat pancreatic acini. Acta Pharmacol Sin. 2000;21:377-380. [PubMed] |
| 24. | Burdakov D, Galione A. Two neuropeptides recruit different messenger pathways to evoke Ca2+ signals in the same cell. Curr Biol. 2000;10:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Petersen OH, Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987;67:1054-1116. [PubMed] |
| 26. | Tsunoda Y, Tashiro Y. Distinct characteristics of receptor-operated Ca2+ influx and refilling in pancreatic acinar cells. Biochem Biophys Res Commun. 1999;256:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Cui ZJ, Kanno T. Photodynamic triggering of calcium oscillation in the isolated rat pancreatic acini. J Physiol. 1997;504:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Cui ZJ, Habara Y, Wang DY, Kanno T. A novel aspect of photodynamic action: induction of recurrent spikes in cytosolic calcium concentration. Photochem Photobiol. 1997;65:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Cui ZJ. Types of voltage-dependent calcium channels involved in high potassium depolarization-induced amylase secretion in the exocrine pancreatic tumour cell line AR4-2J. Cell Res. 1998;8:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Matthews EK, Cui ZJ. Photodynamic action of rose bengal on isolated rat pancreatic acini: stimulation of amylase release. FEBS Lett. 1989;256:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Matthews EK, Cui ZJ. Photodynamic action of sulphonated aluminium phthalocyanine (SALPC) on isolated rat pancreatic acini. Biochem Pharmacol. 1990;39:1445-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Matthews EK, Cui ZJ. Photodynamic action of sulphonated aluminium phthalocyanine (SALPC) on AR4-2J cells, a carcinoma cell line of rat exocrine pancreas. Br J Cancer. 1990;61:695-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Cui ZJ, Guo LL. Assessing physiological concentrations of endogenous substances in situ by inducing calcium oscillations in vitro. Case of liver. Acta Pharmacol Sin. 2002;23:27-32. [PubMed] |
| 34. | Hashikura S, Satoh Y, Cui ZJ, Habara Y. Photodynamic action inhibits compound 48/80-induced exocytosis in rat peritoneal mast cells. Jpn J Vet Res. 2001;49:239-247. [PubMed] |
| 35. | Cui ZJ, Habara Y, Satoh Y. Photodynamic modulation of adrenergic receptors in the isolated rat hepatocytes. Biochem Biophys Res Commun. 2000;277:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Murai A, Satoh S, Okumura J, Furuse M. Factors regulating amylase secretion from chicken pancreatic acini in vitro. Life Sci. 2000;66:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Satoh Y, Matsuo T, Sogabe H, Itoh H, Tada T, Kinoshita T, Yoshida K, Takaya T. Studies on a novel, potent and orally effective cholecystokinin A antagonist, FK-480. Synthesis and structure-activity relationships of FK-480 and related compounds. Chem Pharm Bull (Tokyo). 1994;42:2071-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Kuno M, Sogabe H, Ito H, Matsuo T, Satoh Y, Motoyama Y, Tanaka H. Augmentation of the inhibitory effect of FK480, a CCK-A receptor antagonist, on pancreatic exocrine secretion by achlorhydria. Pancreas. 1998;17:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Kihara Y, Otsuki M. Different inhibitory effects of the newly developed CCK receptor antagonists FK480 and KSG-504 on pancreatic exocrine and endocrine secretion in the isolated perfused rat pancreas. Pancreas. 1995;10:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Akiyama T, Otsuki M. Characterization of a new cholecystokinin receptor antagonist FK480 in in vitro isolated rat pancreatic acini. Pancreas. 1994;9:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Ito H, Sogabe H, Nakarai T, Sato Y, Tomoi M, Kadowaki M, Matsuo M, Tokoro K, Yoshida K. Pharmacological profile of FK480, a novel cholecystokinin type-A receptor antagonist: comparison to loxiglumide. J Pharmacol Exp Ther. 1994;268:571-575. [PubMed] |
| 42. | Xiong CS. [Effect of toosendanin on the ultrastructure of the rat neuromuscular junction]. Yaoxue Xuebao. 1982;17:407-412. [PubMed] |
| 43. | Xiong CS. [The interaction between toosedanin and botulinum toxin at the neuromuscular junction; an ultrastructural observation]. Yaoxue Xuebao. 1985;20:495-499. [PubMed] |
| 44. | Bernatchez G, Berrou L, Benakezouh Z, Ducay J, Parent L. Role of Repeat I in the fast inactivation kinetics of the Ca (V)2.3 channel. Biochim Biophys Acta. 2001;1514:217-229. [PubMed] |
| 45. | Berjukow S, Marksteiner R, Sokolov S, Weiss RG, Margreiter E, Hering S. Amino acids in segment IVS6 and beta-subunit interaction support distinct conformational changes during Ca (v)2.1 inactivation. J Biol Chem. 2001;276:17076-17082. [PubMed] |
| 46. | Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20:6830-6838. [PubMed] |
| 47. | Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 371] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 48. | Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron. 1999;22:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 659] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 49. | Villarroya M, Olivares R, Ruíz A, Cano-Abad MF, de Pascual R, Lomax RB, López MG, Mayorgas I, Gandía L, García AG. Voltage inactivation of Ca2+ entry and secretion associated with N- and P/Q-type but not L-type Ca2+ channels of bovine chromaffin cells. J Physiol. 1999;516:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Cui ZJ. [Ca2+/calmodulin-dependent protein kinase II and its functions]. Sheng Li Ke Xue Jin Zhan. 1997;28:61-63. [PubMed] |
| 51. | Cui ZJ. Muscarinic stimulation of calcium/calmodulin-dependent protein kinase II in isolated rat pancreatic acini. Zhongguo Yaoli Xuebao. 1997;18:255-258. [PubMed] |
| 52. | Cui ZJ, Gorelick FS, Dannies PS. Calcium/calmodulin-dependent protein kinase-II activation in rat pituitary cells in the presence of thyrotropin-releasing hormone and dopamine. Endocrinology. 1994;134:2245-2250. [PubMed] |
| 53. | Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1432] [Cited by in RCA: 1412] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 54. | Yan Z, Chi P, Bibb JA, Ryan TA, Greengard P. Roscovitine: a novel regulator of P/Q-type calcium channels and transmitter release in central neurons. J Physiol. 2002;540:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Kato A, Ohkubo T, Kitamura K. Algogen-specific pain processing in mouse spinal cord: differential involvement of voltage-dependent Ca (2+) channels in synaptic transmission. Br J Pharmacol. 2002;135:1336-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Rathmayer W, Djokaj S, Gaydukov A, Kreissl S. The neuromuscular junctions of the slow and the fast excitatory axon in the closer of the crab Eriphia spinifrons are endowed with different Ca2+ channel types and allow neuron-specific modulation of transmitter release by two neuropeptides. J Neurosci. 2002;22:708-717. [PubMed] |
| 57. | Santafé MM, Garcia N, Lanuza MA, Uchitel OD, Salon I, Tomàs J. Decreased calcium influx into the neonatal rat motor nerve terminals can recruit additional neuromuscular junctions during the synapse elimination period. Neuroscience. 2002;110:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |