Published online Oct 15, 2002. doi: 10.3748/wjg.v8.i5.913
Revised: April 15, 2002
Accepted: April 20, 2002
Published online: October 15, 2002
AIM: To synthesize dexamethasone-succinate-dextran (DSD) conjugate and to evaluate the potentiality of DSD for the treatment of inflammatory bowel diseases.
METHODS: Dexamethasone was attached to dextran (average molecular weight = 70400 Dalton) using succinate anhydride in an anhydrous environment catalyzed by 4-dimethylaminopyridine and 1,1’-carbonyldiimidazole. The chemical structure of DSD was identified by UV, IR and NMR, and the in vivo drug release behavior of this prodrug was investigated after oral administration of DSD suspension.
RESULTS: The DSD conjugate was obtained in two steps and the content of dexamethasone in DSD was 11.28%. The dextran prodrug was stable in rat stomach and small intestine and negligibly absorbed from these tracts. Four to nine hours after the oral administration, most of the prodrug (> 95%) had moved to the cecum and colon, and was easily hydrolyzed by an endodextranase. Recover of dexamethasone from colon and cecum after administration of DSD conjugate was 6-12 folds higher than the recovery after administration of unmodified dexamethasone (t = 2.74, P < 0.05). The preferential release of free dexamethasone in cecum and colon over that in the small intestine was statistically significant (t = 2.27, P < 0.05).
CONCLUSION: The results of this study indicate that dextran conjugates may be useful in selectively delivering glucocorticoids to the colon.
- Citation: Pang YN, Zhang Y, Zhang ZR. Synthesis of an enzyme-dependent prodrug and evaluation of its potential for colon targeting. World J Gastroenterol 2002; 8(5): 913-917
- URL: https://www.wjgnet.com/1007-9327/full/v8/i5/913.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i5.913
Inflammatory bowel diseases, which include ulcerative colitis and Crohn's disease are currently treated with glucocoticoids and other anti-inflammatory agents[1,2]. For a steroidal anti-inflammatory drug, e.g. dexamethasone or prednisolone, a long-term administration would produce systemic side effects, including adrenosuppression, Cushinoid symptoms, immunosuppression, and diabetes. In this case, it is desirable to localize the release of dexamethasone insofar as possible to the afflicted sites in the colon. Release of drug in the proximal GI tract should be avoided to circumvent absorption from the small intestine, and consequent drug wastage and systemic side effects[2]. Because of the unique physiological characteristics of the large intestine, drug delivery to the colon can be achieved in different ways, including pH dependent approaches utilizing the changes in pH along the GI tract[3-12], coated dosage forms[13-17], time-controlled or pulsatile release systems[18-24], pressure-controlled colon delivery systems[25-30], coating drugs with bacterially degradable polymers[31-40], and delivery of drugs as prodrugs[41-47].
The bacterial count in the colon is higher than that in the preceding sections of the GI tract by many orders of magnitude in humans and other animals. Enzymes of the colonic bacteria can specifically degrade some kind of polysaccharides and azo-polymers or break the chemical bonds between the parent drug and the carrier, and then the pharmacological active component can be released from natural and synthetic prodrugs. The most important issue for this approach is a selection of the functional groups that can survive the passage through stomach and small intestine, but are degraded by enzymes of the colonic microflora thus specifically releasing the drug into the colon[2].
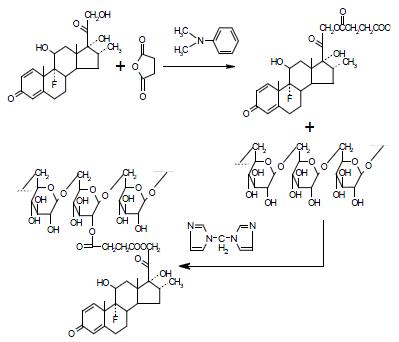
This project used dexamethasone as the model drug to synthesize a prodrug via a succinate tetracarbon-bridge that links the parent drug to the dextran carrier. Compared with unmodified dexamethasone, dexamethasone-succinate-dextran conjugate is more hydrophilic and has a larger molecular weight, which may decrease its possibility of being absorbed into the systemic circulation through the small intestinal epithelial cells. When it arrives to the colon, the dextran structure is hydrolyzed quickly by endogenous dextranase and then the esterase breaks the ester bond to release the dexamethasone. Distributions of dexamethasone in plasma and luminal contents were investigated after gastric intubation of DSD suspension or equivalent dose of dexamethasone to male SD rats.
Dexamethasone was purchased from Tianjin Pharmacy Ltd., China.4-dimethylaminopyridine (DMPA), 1,1'-carbonyldiimidazole and dextran (weight-average molecular weight = 70400 Dalton) were obtained from Sigma Chemical Company, St. Louis, MO. Succinate anhydride was purchased from Beijing Medicine Corporation, China. Molecular sieve (5A) was obtained from Shitian Chemical Ind. China.
Synthesis of dexamethasone-dextran conjugate Dexamethasone 3.98 g, succinate anhydride 1.27 g and 4-dimethylaminopyridine 1.55 g were dissolved in 400 mL anhydrous acetone over 5'-molecular sieves. The reaction solution was stirred at 25 °C for 30 min, and the resulting solution was evaporated in a rotary evaporator to produce light yellow solid. After the solid was dissolved in anhydrous ethanol, distilled water was added to achieve a solution of ethanol and water (29:71 v/v). The solution was kept at -4 °C for 48 h to crystallize and filtered under reduced pressure. The resulting crystals were dried in a P2O5 drying pistol with refluxing of 95% ethanol under vacuum (10 mm Hg) for 24 h to produce dexamethasone succinate hemiester (DS). The yield is 85.28% ± 4.57%.
3.08 g of DS and 1.78 g of 1,1'-carbonyldiimidazole were dissolved in 15 mL of anhydrous dimethyl sulfoxide (DMSO). The reaction was run at 25 °C with stirring for 30 min. Then a solution of dextran in anhydrous DMSO (200 mL) and triethylamine (17.5 mL) was added, and the mixture was stirred at 25 °C for 21 h. The dextran conjugate was precipitated by adding 300 mL of ethanol/ether (50:50 v/v) to the DMSO solution with stirring. The resulting polymer was dispersed in ethanol again and filtered under a stream of dry nitrogen. The precipitate was collected by filtration under reduced pressure, then washed with anhydrous ether three times to produce DSD white powder (yield: 81.27% ± 5.09%): UV λmax: 242 nm (ε14500); IR (KBr): 3420 (OH), 2930 (CH2), 1740 (C = O), 1660 (C = C), 1020 (C-O-C) 898 cm-1; 1HNMR (DEXO-d6): δ7.310, 7.285 (d, 1H, C-1), 6.233, 6.207 (d, 1H, C-2), 6.002 (s, 1H, C-4), 3.488, 3.508, 3.623, 3.742, 4.668 (s, 1H, C-5', C-4', C-3', C-2', C-1'), 3.202 (s, 2H, C-6), 2.054 (s, 2H, C-21), 1.464 (s, 3H, C-19), 0.860 (s, 3H, C-18), 0.758, 0.774 (d, 3H, C-16).
The content of dexamethasone in DSD was measured by HPLC after alkaline hydrolysis.
Preparation of DSD granules and DSC test To evaluate the potential colon specificity of DSD in vivo test, granules of DSD or dexamethasone were prepared with the following ingredients: DSD or dexamethasone, cornstarch and lactose (5:50:45). A wet granulation method was applied. The granules were partially dissolved and suspended in water before dosing. Before granulation, all the ingredients were subjected to Differential Scanning Calorimetry (DSC). Measurements were performed on a calorimeter DSC7 connected to a Thermal Analysis Data Station 3700 (Perkin-Elmer, Germany). Five mg of bulk materials were accurately weighed into standard aluminum pans. Thermograms were recorded from 303 to 573 K at a heating rate of 10 K·min-1.
In vivo test Male SD rats (weighing about 150 g) were fed a standard diet (R-2, Chengdu) and were fasted for 18 h prior to drug administration with free access to drinking water. The rats were divided into the test group and control group randomly. Each group was subdivided further into seven subgroups. The test groups were administered with suspension of DSD (equivalent to 3 mg of dexamethasone per kg of rat body weight) by gastric intubation, and the control groups were administered with suspension of dexamethasone. After the drug administration, blood samples of the test subgroups and the control subgroups were collected at each predetermined time (1, 3, 4, 5, 6, 7, 9 h). Then the rats were sacrificed by decapitation and the stomach, proximal small intestine (PSI), distal small intestine (DSI), cecum and colon were removed. The contents of the GI tract were removed by gently squeezing the GI segments. The separated contents and tissues were quickly frozen to -20 °C and stored until analysis. Rat blood samples were collected and centrifuged at 700 g for 10 min. The plasma was frozen to -20 °C and stored until analysis was performed.
Analysis The frozen intestinal contents were thawed, weighed, and diluted to 50% (w/v) with phosphate buffer (pH6.8). The suspended samples were homogenized by vortexing, and then 0.5 g of the diluted contents was placed in a 5-mL centrifuge tube. 200 μL of isotonic phosphate buffer (pH2), 100 μL of internal standard solution (0.1336 mg·mL-1) and dexamethasone solution in different concentrations were added to the 5-mL centrifuge tube. The samples were extracted with acetic ester (3 mL) by vortexing for 2 min. After centrifuging for 10 min at 1000 g, 2 mL of the organic phase was removed and evaporate at 45 °C under vacuum. The residue was redissolved in mobile phase solution and centrifuged for 10 min at 1000 g. 20 μL of the supernatant fluid was subjected to HPLC analysis under the following conditions: Shimadzu CTO-10A system controller, LC-10AT pumps, SPD-10A variable wavelength detector, a Shimpack CLC C18 column (5 μm, 4.6 × 150 mm). The mobile phase consisted of 35% acetonitrile and 65% buffer (50 mM trisodium citrate adjusted to pH4.6 with phosphoric acid). A flow rate of 1 mL•min⁻¹ and a detection wavelength of 241 nm were used. Prednisolone acetate was used as the internal standard. The plasma samples were treated by the same method described above.
DSD was prepared in two steps with a modified Mcleod reaction[2]. It is essential to keep the reaction continuing under the anhydrous condition to ensure high yield. Succinate anhydride was coupled to the dexamethasone hydroxyl group in anhydrous acetone in the presence of 4-dimethylaminopyridine to produce hemiester. Then the hemiester was coupled to dextran in DMSO using 1,1'-carbonyldiimidazole as catalyzer (Figure 1).
The chemical structure was identified by 1HNMR and IR, confirming the procedure of scheme 1. The content of dexamethasone in DSD was 11.28 equals to about 20 dexamethasone (molecular weight = 392 Dalton) molecules were coupled to one dextran molecule (average molecular weight = 70400 Dalton).
Study on interaction between the supplementary ingredients of suspension and DSD was performed by DSC. The thermogram displayed two transition peaks at 343 K and 561 K corresponding to DSD, and another two peaks at 420 K and 486 K corresponding to mixed ingredients. No new transition peak was observed when the physical mixture of DSD and ingredients were subjected to DSC, indicating that there was no interaction between DSD and the supplementary ingredients.
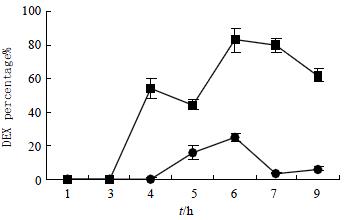
The recovery of free dexamethasone from rat blood and GI tract at various times following oral administration of DSD suspensions is shown in Table 1. During the whole observation period (0-9 h), no dexamethasone was detected in blood. This observation indicated that DSD conjugate was so stable that it could not be degraded in upper GI tract and could not be absorbed into blood. Three hours after dosing, only very small amount (< 3% of total recovery) of dexamethasone was detected in small intestine in spite of the high level of esterase in small intestine. After 6 h, the recovery of dexamethasone in small intestine further decreased. At the same time, a large portion (> 95% of total recovery) of the prodrug reached the cecum and colon intact.
| th | Tμg | Recovery of dexamethasone (%) | |||||
| B | S | PSI | DSI | Ce | Co | ||
| 1 | 5.2 | nd | 74 | 26 | nd | nd | nd |
| 3 | 7.3 | nd | 54 | nd | 46 | nd | nd |
| 4 | 14 | nd | 8.8 | 6.6 | 10.2 | 54 | 20.5 |
| 5 | 27.6 | nd | 9.2 | 5.0 | 13.4 | 54.4 | 18.0 |
| 6 | 133 | nd | 4.0 | 0.27 | 2.2 | 83 | 10.2 |
| 7 | 128 | nd | 4.5 | nd | 0.557 | 80 | 15.2 |
| 9 | 105 | nd | 12.4 | 3.49 | 3.72 | 62 | 18.2 |
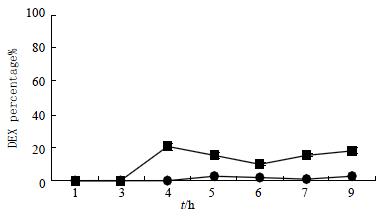
Control experiments in which unmodified dexamethasone was administered showed that dexamethasone was absorbed primarily from the small intestine and the blood concentration of dexamethasone was much higher than test groups. Meanwhile, very small amount of dexamethasone was observed either in the cecum or in the colon (Table 2).
| th | Tμg | Recovery of dexamethasone (%) | |||||
| B | S | PSI | DSI | Ce | Co | ||
| 1 | 1076 | 8.9 | 54 | 15.8 | 20.8 | 0.05 | nd |
| 3 | 857 | 4.58 | 94.6 | 0.05 | 1.08 | nd | nd |
| 4 | 390 | 26 | 58 | 10.2 | 5.0 | 0.40 | nd |
| 5 | 230 | 36 | 22 | 1.19 | 4.2 | 16 | 2.56 |
| 6 | 203 | 33 | 30 | 6.7 | 3.63 | 24.7 | 2.25 |
| 7 | 139 | 39 | 49 | 2.02 | 5.6 | 3.74 | 0.79 |
| 9 | 134 | 40 | 42 | 1.52 | 7.8 | 6.0 | 3.1 |
The dexamethasone recoveries from cecum and colon after the oral administration of DSD suspensions or dexamethasone suspensions were also shown graphically (Figure 2 and Figure 3). It was obvious that the recovery of test groups from cecum and colon after administration were higher than that of control groups by 6-12 folds (t = 2.74, P < 0.05).
The specificity of dexamethasone release was further evaluated by comparing the amount of free dexamethasone recovered in the small intestine with that in the colon in the test group. A paired t-test indicated that the preferential release of free dexamethasone in cecum and colon over that in the small intestine was statistically significant (t = 2.27, P < 0.05). Meanwhile, a similar analysis in the control group showed that the difference between the dexamethasone concentration in the colon and that in the small intestine was not statistically significant.
The bacterial count in the colon is much higher than that in upper GI tract[2]. The colonic micro flora produce a variety of enzymes, including azoreductase, various glycosidases and amidases, which are not present in the stomach or the small intestine. Therefore, enzyme dependent drug release, which relies on the existence of enzyme-producing microorganisms in the colon, could be used to deliver drug to the colon after enzymatic cleavage of degradable carrier bonds and premature drug release does not occur in this case.
Besides treating inflammatory bowel diseases, colon-specific drug delivery system might be useful in other situations. The delivery of certain antineoplastic agents to the colon might be beneficial in controlling colon cancer[48]. Enzyme prodrug gene therapy for colon cancer is also investigated by several researchers[49,50]. Antibiotics might be delivered specifically to the colon via cyclodextrin carriers[51-53]. In each of these cases, colon-specific delivery would allow the use of higher doses of potent agents with fewer systemic side effects.
The present results showed that the ester type prodrugs of dexamethasone/dextran release dexamethasone preferentially on cecal and colonic contents after the hydrolysis of dextran to small oligosaccharides, suggesting that dextran could serve as a new class of colon-specific drug carrier. The dextran conjugate survives the passage through upper GI tract although the high level of esterase in small intestine, indicating that dextran protects ester bond from hydrolysis by esterase. This result, together with the observation mentioned above, suggests that bacterial enzymes in the colon are responsible for hydrolysis of dextran conjugates. When DSD reached the colon, dextran was completely hydrolyzed into smaller oligosaccharides and exposed the ester bonds to esterase, which led to the rapid release of dexamethasone.
In summary, a colon-specific drug-delivery system has been developed based on drug-dextran conjugation and the unique glycosidase activity of the colonic microflora. Colonic drug delivery can be achieved with carriers by making prodrugs that survive the passage through stomach and small intestine, but the active moiety is released by enzymes specifically produced in colon.
Edited by Bo XN
| 1. | Jiang XL, Cui HF. An analysis of 10218 ulcerative colitis cases in China. World J Gastroenterol. 2002;8:158-161. [PubMed] |
| 2. | McLeod AD, Friend DR, Tozer TN. Glucocorticoid-dextran conjugates as potential prodrugs for colon-specific delivery: hydrolysis in rat gastrointestinal tract contents. J Pharm Sci. 1994;83:1284-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Rudolph MW, Klein S, Beckert TE, Petereit H, Dressman JB. A new 5-aminosalicylic acid multi-unit dosage form for the therapy of ulcerative colitis. Eur J Pharm Biopharm. 2001;51:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Khan MZ, Prebeg Z, Kurjaković N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation Of drug release using Eudragit L100-55 and Eudragit S100 combinations. J Control Release. 1999;58:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Gupta VK, Beckert TE, Price JC. A novel pH- and time-based multi-unit potential colonic drug delivery system. I. Development. Int J Pharm. 2001;213:83-91. [PubMed] |
| 6. | Gupta VK, Assmus MW, Beckert TE, Price JC. A novel pH- and time-based multi-unit potential colonic drug delivery system. II. Optimization of multiple response variables. Int J Pharm. 2001;213:93-102. [PubMed] |
| 7. | Ishibashi T, Pitcairn GR, Yoshino H, Mizobe M, Wilding IR. Scintigraphic evaluation of a new capsule-type colon specific drug delivery system in healthy volunteers. J Pharm Sci. 1998;87:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Nykänen P, Krogars K, Säkkinen M, Heinämäki J, Jürjensson H, Veski P, Marvola M. Organic acids as excipients in matrix granules for colon-specific drug delivery. Int J Pharm. 1999;184:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Cole ET, Scott RA, Connor AL, Wilding IR, Petereit HU, Schminke C, Beckert T, Cadé D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int J Pharm. 2002;231:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Rodríguez M, Vila-Jato JL, Torres D. Design of a new multiparticulate system for potential site-specific and controlled drug delivery to the colonic region. J Control Release. 1998;55:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Lorenzo-Lamosa ML, Remuñán-López C, Vila-Jato JL, Alonso MJ. Design of microencapsulated chitosan microspheres for colonic drug delivery. J Control Release. 1998;52:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 248] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Rodriguez M, Antúnez JA, Taboada C, Seijo B, Torres D. Colon-specific delivery of budesonide from microencapsulated cellulosic cores: evaluation of the efficacy against colonic inflammation in rats. J Pharm Pharmacol. 2001;53:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Leopold CS, Eikeler D. Basic coating polymers for the colon-specific drug delivery in inflammatory bowel disease. Drug Dev Ind Pharm. 2000;26:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Cavalcanti OA, Van den Mooter G, Caramico-Soares I, Kinget R. Polysaccharides as excipients for colon-specific coatings. Permeability and swelling properties of casted films. Drug Dev Ind Pharm. 2002;28:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Nykänen P, Lempää S, Aaltonen ML, Jürjenson H, Veski P, Marvola M. Citric acid as excipient in multiple-unit enteric-coated tablets for targeting drugs on the colon. Int J Pharm. 2001;229:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Villar-López ME, Nieto-Reyes L, Anguiano-Igea S, Otero-Espinar FJ, Blanco-Méndez J. Formulation of triamcinolone acetonide pellets suitable for coating and colon targeting. Int J Pharm. 1999;179:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Tozaki H, Fujita T, Komoike J, Kim SI, Terashima H, Muranishi S, Okabe S, Yamamoto A. Colon-specific delivery of budesonide with azopolymer-coated pellets: therapeutic effects of budesonide with a novel dosage form against 2,4,6-trinitrobenzenesulphonic acid-induced colitis in rats. J Pharm Pharmacol. 1999;51:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Fukui E, Miyamura N, Kobayashi M. An in vitro investigation of the suitability of press-coated tablets with hydroxypropylmethylcellulose acetate succinate (HPMCAS) and hydrophobic additives in the outer shell for colon targeting. J Control Release. 2001;70:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Fukui E, Miyamura N, Uemura K, Kobayashi M. Preparation of enteric coated timed-release press-coated tablets and evaluation of their function by in vitro and in vivo tests for colon targeting. Int J Pharm. 2000;204:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Sangalli ME, Maroni A, Zema L, Busetti C, Giordano F, Gazzaniga A. In vitro and in vivo evaluation of an oral system for time and/or site-specific drug delivery. J Control Release. 2001;73:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Ishibashi T, Ikegami K, Kubo H, Kobayashi M, Mizobe M, Yoshino H. Evaluation of colonic absorbability of drugs in dogs using a novel colon-targeted delivery capsule (CTDC). J Control Release. 1999;59:361-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Sangalli ME, Maroni A, Busetti C, Zema L, Giordano F, Gazzaniga A. In vitro and in vivo evaluation of oral systems for time and site specific delivery of drugs (Chronotopic technology). Boll Chim Farm. 1999;138:68-73. [PubMed] |
| 23. | Yang L, Chu JS, Fix JA. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm. 2002;235:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 333] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Hebden JM, Wilson CG, Spiller RC, Gilchrist PJ, Blackshaw E, Frier ME, Perkins AC. Regional differences in quinine absorption from the undisturbed human colon assessed using a timed release delivery system. Pharm Res. 1999;16:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Takaya T, Niwa K, Muraoka M, Ogita I, Nagai N, Yano R, Kimura G, Yoshikawa Y, Yoshikawa H, Takada K. Importance of dissolution process on systemic availability of drugs delivered by colon delivery system. J Control Release. 1998;50:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Muraoka M, Hu Z, Shimokawa T, Sekino S, Kurogoshi R, Kuboi Y, Yoshikawa Y, Takada K. Evaluation of intestinal pressure-controlled colon delivery capsule containing caffeine as a model drug in human volunteers. J Control Release. 1998;52:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Takaya T, Sawada K, Suzuki H, Funaoka A, Matsuda K, Takada K. Application of a colon delivery capsule to 5-aminosalicylic acid and evaluation of the pharmacokinetic profile after oral administration to beagle dogs. J Drug Target. 1997;4:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Hu Z, Kimura G, Mawatari S, Shimokawa T, Yoshikawa Y, Takada K. New preparation method of intestinal pressure-controlled colon delivery capsules by coating machine and evaluation in beagle dogs. J Control Release. 1998;56:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Shibata N, Ohno T, Shimokawa T, Hu Z, Yoshikawa Y, Koga K, Murakami M, Takada K. Application of pressure-controlled colon delivery capsule to oral administration of glycyrrhizin in dogs. J Pharm Pharmacol. 2001;53:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Jeong YI, Ohno T, Hu Z, Yoshikawa Y, Shibata N, Nagata S, Takada K. Evaluation of an intestinal pressure-controlled colon delivery capsules prepared by a dipping method. J Control Release. 2001;71:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Chavan MS, Sant VP, Nagarsenker MS. Azo-containing urethane analogues for colonic drug delivery: synthesis, characterization and in-vitro evaluation. J Pharm Pharmacol. 2001;53:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Tozaki H, Nishioka J, Komoike J, Okada N, Fujita T, Muranishi S, Kim SI, Terashima H, Yamamoto A. Enhanced absorption of insulin and (Asu (1, 7)) eel-calcitonin using novel azopolymer-coated pellets for colon-specific drug delivery. J Pharm Sci. 2001;90:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Tozaki H, Fujita T, Odoriba T, Terabe A, Okabe S, Muranishi S, Yamamoto A. Validation of a pharmacokinetic model of colon-specific drug delivery and the therapeutic effects of chitosan capsules containing 5-aminosalicylic acid on 2,4,6-trinitrobenzenesulphonic acid-induced colitis in rats. J Pharm Pharmacol. 1999;51:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Macleod GS, Fell JT, Collett JH, Sharma HL, Smith AM. Selective drug delivery to the colon using pectin: chitosan: hydroxypropyl methylcellulose film coated tablets. Int J Pharm. 1999;187:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Brøndsted H, Andersen C, Hovgaard L. Crosslinked dextran--a new capsule material for colon targeting of drugs. J Control Release. 1998;53:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Kakoulides EP, Smart JD, Tsibouklis J. Azocrosslinked poly (acrylic acid) for colonic delivery and adhesion specificity: in vitro degradation and preliminary ex vivo bioadhesion studies. J Control Release. 1998;54:95-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Stubbe B, Maris B, Van den Mooter G, De Smedt SC, Demeester J. The in vitro evaluation of 'azo containing polysaccharide gels' for colon delivery. J Control Release. 2001;75:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Adkin DA, Kenyon CJ, Lerner EI, Landau I, Strauss E, Caron D, Penhasi A, Rubinstein A, Wilding IR. The use of scintigraphy to provide "proof of concept" for novel polysaccharide preparations designed for colonic drug delivery. Pharm Res. 1997;14:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Krishnaiah YS, Veer Raju P, Dinesh Kumar B, Bhaskar P, Satyanarayana V. Development of colon targeted drug delivery systems for mebendazole. J Control Release. 2001;77:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Krishnaiah YS, Satyanarayana S, Rama Prasad YV, Narasimha Rao S. Gamma scintigraphic studies on guar gum matrix tablets for colonic drug delivery in healthy human volunteers. J Control Release. 1998;55:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Davaran S, Hanaee J, Khosravi A. Release of 5-amino salicylic acid from acrylic type polymeric prodrugs designed for colon-specific drug delivery. J Control Release. 1999;58:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Maris B, Verheyden L, Van Reeth K, Samyn C, Augustijns P, Kinget R, Van den Mooter G. Synthesis and characterisation of inulin-azo hydrogels designed for colon targeting. Int J Pharm. 2001;213:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Lee JS, Jung YJ, Kim YM. Synthesis and evaluation of N-acyl-2- (5-fluorouracil-1-yl)-D, L-glycine as a colon-specific prodrug of 5-fluorouracil. J Pharm Sci. 2001;90:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Jung YJ, Lee JS, Kim YM. Colon-specific prodrugs of 5-aminosalicylic acid: synthesis and in vitro/in vivo properties of acidic amino acid derivatives of 5-aminosalicylic acid. J Pharm Sci. 2001;90:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Sinha VR, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm. 2001;224:19-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 503] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 46. | Sinha VR, Kumria R. Colonic drug delivery: prodrug approach. Pharm Res. 2001;18:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Goto M, Okamoto Y, Yamamoto M, Aki H. Anti-inflammatory effects of 5-aminosalicylic acid conjugates with chenodeoxycholic acid and ursodeoxycholic acid on carrageenan-induced colitis in guinea-pigs. J Pharm Pharmacol. 2001;53:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Ichinose K, Tomiyama N, Nakashima M, Ohya Y, Ichikawa M, Ouchi T, Kanematsu T. Antitumor activity of dextran derivatives immobilizing platinum complex (II). Anticancer Drugs. 2000;11:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Wang S, Low PS. Folate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cells. J Control Release. 1998;53:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Kievit E, Bershad E, Ng E, Sethna P, Dev I, Lawrence TS, Rehemtulla A. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59:1417-1421. [PubMed] |
| 51. | Minami K, Hirayama F, Uekama K. Colon-specific drug delivery based on a cyclodextrin prodrug: release behavior of biphenylylacetic acid from its cyclodextrin conjugates in rat intestinal tracts after oral administration. J Pharm Sci. 1998;87:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Yano H, Hirayama F, Arima H, Uekama K. Prednisolone-appended alpha-cyclodextrin: alleviation of systemic adverse effect of prednisolone after intracolonic administration in 2,4,6-trinitrobenzenesulfonic acid-induced colitis rats. J Pharm Sci. 2001;90:2103-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Yano H, Hirayama F, Arima H, Uekama K. Preparation of prednisolone-appended alpha-, beta- and gamma-cyclodextrins: substitution at secondary hydroxyl groups and in vitro hydrolysis behavior. J Pharm Sci. 2001;90:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |