INTRODUCTION
Cytochrome P450 (CYP) is a heme-containing enzyme widely distributed from bacteria to mammals, and catalyzes the oxidative and reductive metabolism of a wide variety of compounds including endogenous as well as exogenous compounds. Mammalian CYP present in liver microsomes is characteristic of its nature in metabolizing exogenous compounds including drugs, pesticides, environmental pollutants, and carcinogens[1]. Mammals contain at least 17 distinct CYP gene families that together code for an estimated 50-60 individual CYP genes in any given species[2]. The human CYP2C subfamily comprises four members, CYP2C8, CYP2C9, CYP2C18 and CYP2C19[3], accounting for 20% of the total CYP in human liver. CYP2C18 mRNA was found in liver, albeit at mean levels 7-8-fold lower than those of mRNAs encoding CYP2C8 and CYP2C9[4,5]. The cDNA encoding human CYP2C18 has been characterized, but the protein has not been purified from liver, and very little is known regarding the specific substrate of CYP2C18[4].
The combination of gene technology and cell culture technology has provided new opportunities for studying proteins because any gene from any species encoding a protein may be cloned and expressed in bacterial, yeast, or mammalian cell in a defined way[6-11]. This approach in drug metabolism is of particular importance because some of the enzymes are difficult to purify and to prepare in sufficient quantities, for its low expression levels, organ-specificity of its expression, or low abundance of native organ material. These restrictions apply especially for human enzymes. The heterologous expression of the cDNA bypasses these restrictions[12]. The human CYP2C18 cDNA had been expressed in yeast[13,14], COS-1 cells[3], lymphoblast cells[15], and human liver epithelial cells THLE[16]. Several cell lines stably expressing human CYP1A1[17], CYP2B6[17], CYP2A6[18], CYP3A4[19], CYP2C9[20] and a phase II metabolism enzyme UDP-glucuronosyltransferase, UGT1A9[21] have been established in our laboratory. In this study human CYP2C18 cDNA was amplified with reverse transcription-polymerase chain reaction (RT-PCR), and a transgenic cell line stably expressing CYP2C18 was established. In the cloning process, we have identified a spliced variant of CYP2C18 with exon 5 missing.
MATERIALS AND METHODS
Materials
Restriction endonucleases, moloney murine leukemia virus (M-MuLV) reverse transcriptase were supplied by MBI Fermentas AB, Lithuania. PCR primers, DNA sequence primers, random hexamer primer, Taq plus I, and dNTPs were synthesized or supplied by Shanghai Sangon Biotechnology Corp. DNA sequencing kit was purchased from Perkin-Elmer Corp. The TRIzol Reagent, G418, Minimum Essential Media (MEM) and newborn bovine calf sera were from Gibco. NADPH was from Roche molecular biochemicals. Diethyl pyrocarbonate (DEPC), tolbutamide and hydroxytolbutamide were purchased from Sigma Chemical Company. T4 DNA ligase and pGEM-T vector system were from Promega. Other chemical reagents used are all of analytical purity from the commercial sources.
Methods
Cloning of human CYP2C18 cDNA from a Chinese human liver The total RNA was extracted from a surgical specimen of human liver with TRIzol reagent according to the manufacture's instructions. The RT-PCR amplifications were described before[20]. Two specific 28 mer oligonucleotide PCR primers were designed according to the mRNA sequence of CYP2C18 reported by Romkes et al[3] (GenBank accession no. M61856, J05326). The sense primer corresponding to base position 38 to 65 was 5'-TTATCTTCTTCAGCTAGCCAATGTTCAT-3' with a restriction site of Nhe I, and the anti-sense primer, corresponding to the base position from 1681 to 1708, was 5'-TGACAGCACTCGAGCAGCCAAACTATCT-3' with a restriction site of Xho I. The PCR was performed at 94 °C 2 min, then 35 cycles of 94 °C 60 s, 55 °C 60 s, 72 °C 2 min, and lastly 72 °C 10 min. An aliquot (10 mL) from the PCR was subjected to electrophoresis in a 1% agarose gel stained with ethidum bromide.
Construction of recombinant pGEM-CYP2C18 and sequencing of CYP2C18 cDNA[22] The PCR products were ligated with pGEM-T vector, and transformed to the E. coli DH5α. Several cDNAs of CYP2C18 cloned in pGEM-T were sequenced on both strands by dideoxy chain-termination method marked with BigDye with primers of T7 and SP6 promoters and two specific primers of 5'-GGACATGAGCAAATCCTTA-3' (343-361), and 5'-TGGGGATGAGGTAGTTTTTG-3' (1327-1346). The termination products were resolved and detected using an automated DNA sequencer (Perkin-Elmer-ABI Prism 310).
Construction of the pREP9 based expression plasmid for CYP2C18[22] The Nhe I/Xho I fragment having the total span of human CYP2C18 cDNA and the correctly deduced amino acids sequence in pGEM-CYP2C18 was subcloned to a mammalian expression vector pREP9 (Invitrogen). The recombinant was transformed to E. coli Top 10, and screened by ampicillin resistant. The recombinant was identified by restriction mapping.
Transfection and selection[20,22] Chinese hamster lung (CHL) cells were transfected with the resultant recombinant plasmid, pREP9-CYP2C18, using a modified calcium phosphate method. A tansgenic cell line named CHL-CYP2C18 was established.
Preparation of postmitochondrial supernant (S9) of CHL-CYP2C18 The procedure of preparation of the S9 fraction was described before[20]. The protein in S9 was determined by the Lowry's method, with bovine serum albumin as standard. CYP was measured spectrally using the method of Johannesen et al[23].
Tolbutamide hydroxylase assays[20,24,25] The CYP2C18 tolbutamide hydroxylase activity of S9 was determined by high performance liquid chromatography (HPLC) as described before[20].
RESULTS
Construction of recombinants and detection of an exon 5 skipping in transcripts of the human CYP2C18 gene
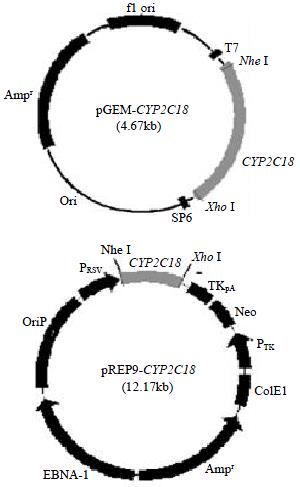
The recombinant of pGEM-CYP2C18 (Figure 1) was constructed with the human CYP2C18 cDNA inserted into the cloning site of pGEM-T vector. Selection and identification of the recombinant was carried out by Nhe I/Xho I endonuclease digestion and agarose gel electorphoresis (Figure 2). Two cloned cDNA segments were sequenced completely. Comparing with the cDNA sequence reported by Romkes et al[3] (GenBank accession no. M61856, J05326), one has two base differences, 222T > C, 828C > T, while the encoding amino acid sequence was the same, I74 and H276. However, another cDNA clone was found to have only 8 exons (Figure 3). There is no base difference between this cDNA and the wild type one except the missing exon 5.
Figure 1 Scheme of recombinants of pGEM-CYP2C18 and pREP9-CYP2C18
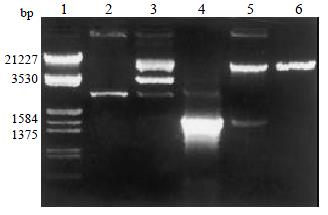
Figure 2 Electrophoresis identification of recombinants of pGEM-CYP2C18 and pREP9-CYP2C18.
Lane 1: Marker (λ/EcoR I and Hind III); 2: pGEM-T vector; 3: Recombinant of pGEM-CYP2C18 digested by Nhe I and Xho I (incompleted digestion); 4: PCR products of CYP2C18 (1.67 kb); 5: Recombinant of pREP9-CYP2C18 digested by Nhe I and Xho I; 6: pREP9 vector.
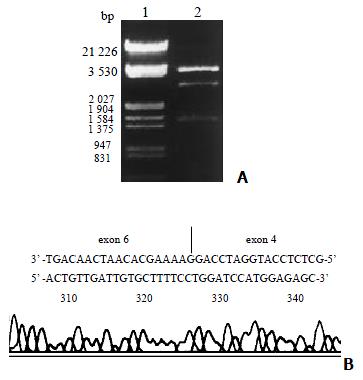
Figure 3 Electrophoresis and sequencing identification of a CYP2C18 clone with exon 5 missing.
A: Electrophoresis identification of a CYP2C18 clone with an exon 5 being skipped; Lane 1: Marker (λ/EcoR I and Hind III); 2: Recombinant of pGEM-CYP2C18 digested by Nhe I and Xho I (incompleted digestion); B: Partial sequencing of an CYP2C18 cDNA clone with an exon 5 being skipped. The upper sequence represent the sense strand and the underside sequence represent the anti-sense strand being sequenced.
The Nhe I/Xho I fragment (1.65 kb) containing the complete CYP2C18 cDNA was subcloned into the Nhe I/Xho I site of mammalian expression vector pREP9 (Figure 1). Selection and identification of the recombinant was carried out by Nhe I/Xho I endonuclease digestion and agarose gel electrophoresis (Figure 2). The resulting plasmid was designated as pREP9-CYP2C18 and contains the entire coding region, along with 162 bp of the 5’ and 36 bp of the 3’ untranslation region of the CYP2C18 cDNA, respectively.
Establishment of transgenic cell lines with CYP2C18 enzyme activity
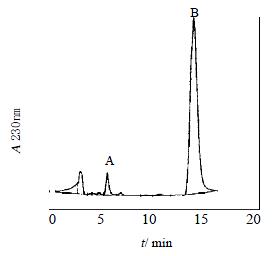
CHL cells were transfected with pREP9-CYP2C18, and selected with G418. The surviving clones were propagated and the cell line termed CHL-CYP2C18 was established. The tolbutamide hydroxylase activity of CYP2C18 in S9 of CHL-CYP2C18 cells was assayed by HPLC. A typical elution profile of hydroxytolbutamide in extracts was shown (Figure 4).
Figure 4 Representative chromatogram of extracts.
A Shim-pack CLC-ODS column (15 × 0.6 cm i.d.) was used. The mobile phase was constituted with 0.05% phosphoric acid (pH2.6), acetonitrile (6:4/V:V) with the flow rate of 1 mL. min-1. Hydroxytolbutamide was monitored at 230 nm. A: hydroxytolbutamide; B: tolbutamide
The CYP2C18 enzyme activity towards tolbutamide was found to be 0.509 ± 0.052 mmol·min-1·g-1 S9 protein or 8.82 ± 0.90 mol·min-1·mol-1 CYP (n = 3), but was not detectable in parental CHL cells. The CYP content was 53.9 pmol·mg-1 S9 protein from CHL-CYP2C18 and no detectable CYP was present in CHL cells.
DISCUSSION
The human CYP2C18 gene is located on chromosome 10q24. Four CYP2C18 alleles have been reported. T385 and M385 alleles exhibited the same regio-and stereoselectivities for warfarin[25]. There was no substantial difference in the ability of the T385 and M385 of CYP2C18 to metabolize mephenytoin[26]. CYP2C18m1, a substitution of 204T > A in exon 2, which creates a stop codon that yields a truncated protein lacking the heme-binding site[27]. CYP2C18mFR, consisting of -460A > T substitution in the 5'-flanking region, the functional property of this mutant allele remains unclear[28]. One of the CYP2C18 cDNA cloned by us belongs to the T385 allele in amino acids sequence. Although there are two base substitutions, 222T > C, 828C > T, the encoding amino acids have not been changed.
To express the functional activity of a CYP, a cell must have adequate heme biosynthetic capabilities and ample intracellular membrane[29]. CYPs also require other enzymatic components for full activity, including the flavoprotein NADPH-P450 oxidoreductase (OR) and, in some cases, cytochrome b5. The OR must interact directly with the CYP to transfer the required two electrons from NADPH. Cytochrome b5 is necessary for increasing electron transfer for certain CYP forms and specific substrates. The CHL is a cell line originally derived from the lung of a newborn female Chinese hamster and has no or very limited activities of CYP enzymes, but has adequate OR and cytochrome b5 levels to support CYP activities.
To achieve high expression levels of CYP2C18, the CYP2C18 cDNA was cloned into the eukaryotic expression vector pREP9, which had previously been used in this laboratory for the expression of human CYP1A1[17], CYP2B6[17], CYP3A4[18], CYP2A6[19], CYP2C9[20] and UGT1A9[21] in CHL cells. The salient feature of this vector has an EBV origin of replication and nuclear antigen (EBNA-1) to allow high-copy episomal replication in mammal cell lines. The Rous sarcoma virus long terminal repeat (RSV LTR) early promoter controls the expression of the CYP2C18 cDNA.
CYP2C18 was expressed at a very low level in human liver[4]. Its protein had not been detected and its mRNA can not be induced by rifampicin or phenobarbital in human livers and cultured primary hepatocytes[5]. But it seems to be a major CYP2C in the skin and the lung judged by its mRNA levels[30]. Its mRNA was found in the brain, uterus, mammary gland, kidney, and duodenum[31]. It has been reported that CYP2C18 can metabolize (S)-mephenytoin[26], tolbutamide[26], warfarin[25], tienilic acid[20], diclofenac[13], aminopyrine[32], and bisphenol A[14] by yeast expression and metabolize all-trans-retinoic acid[15] by lymphoblast expression. Cyclophosphamide (CPA) and ifosfamide can be metabolized by M385 CYP2C18. CYP2C18-M385 plus CYP OR appear to be excellent gene combinations for use with CPA in P450/prodrug activation-based cancer gene therapy[33]. A 2-aroylthiophenes derived from tienilic acid by replacement of its OCH2COOH substituent with O (CH2)3OH function, appears to be a particularly good substrate of CYP2C18[34].
Tolbutamide (1-butyl-3-p-tolylsulfonylurea) is an oral hypoglycemic agent which is being used in the treatment of diabetes. In humans it undergoes CYP-catalyzed hydroxylation of the tolyl methyl group which is the initial and rate-limiting reaction followed by further oxidation by cytosolic dehydrogenases yielding carboxytolbutamide. Overall this pathway accounts for up to 85% of tolbutamide clearance in humans[35]. We used tolbutamide as a substrate for evaluating the expression of human CYP2C18 activity in CHL-CYP2C18 cells. The tolbutamide hydroxylase activity was 0.509 ± 0.052 mmo·min-1·g-1 S9 protein or 8.82 ± 0.90 mol•min⁻¹•mol⁻¹ CYP, which is slightly higher than that of our cloned human CYP2C9: 0.465 ± 0.109 μmol•min⁻¹•g⁻¹ S9 protein or 8.62 ± 2.02 mol•min⁻¹•mol⁻¹ CYP[20]. The tolbutamide hydroxylase activity was reported to be 0.273 ± 0.066[36] or 0.189 ± 0.008[37] mmol•min⁻¹•g⁻¹ of human liver microsomes. Considering the low expression of CYP2C18 in liver, the tolbutamide hydroxylase activity in human liver might be contributed by CYP2C9. CHL-CYP2C18 cells efficiently expressing the CYP2C18 may be a useful tool for further studies of its enzymatic function and mechanism.
Interestingly, we find one of our cloned CYP2C18 cDNAs has only 8 exons by DNA sequencing. This cDNA clone is identical to the wild-type one except the missing exon 5. This is consistent with Zaphiropoulos's report that an exon 5 skipping of human CYP2C18 gene in epidermis[30]. The exon is spliced at the predicted sites indicating that it pre-exists in the mRNA samples and is not a PCR artifact. Transcripts that have skiped CYP2C18 exon 4, exon 4, 5 and 6, or exon 4, 5, 6, and 7 were also identified in epidermis[30]. The splicing process in higher eukaryotes is chracterized by the precise excision of introns that can be longer than 50000 bases and the joining of exons that are rarely over 300 bases. Yet the mechanisms that regulate the splicing process and the generation of alternatively spliced mRNA products are still poorly understood[38]. In addition to alternative splicing in intragenic RNA molecule, chimeric RNA production had been detected in CYP2Cs[39-41] and CYP3As[42] genes. This finding suggests that intergenic mRNA formation may represent a generalized splicing pathway that deepens the complexity of splicing patterns in gene families[42] and the concept of gene transcription may not suffice to include all variations in expressed genomic sequences. Finta and Zaphiropoulos[43] proposed a more general term, "genome transcription".
The difference in enzyme activity between the full length clone and the spliced variant will be studied in the future.