INTRODUCTION
Interactions between the central nervous system (CNS) and the immune system, particularly through neuropeptides, are of growing interest. Due to the abundant autonomic innervation and extensive lymphoid compartment localized in the gastrointestinal tract, a number of neuropeptides with observed and potential immunomodulatory effects have been isolated from gut tissues[1]. These intestinal neuropeptides with immunomodulatory effects include growth hormone[2], somatostatin[3], vasoactive intestinal peptide (VIP)[4,5], calcitonin gene-related peptide (CGRP)[6], neuropeptide Y[7,8], neurotensin[9], and cholecystokinin (CCK)[10].
CCK is discovered initially in the gut with the function of contracting gallbladder, and subsequently localized in the central and peripheral nervous system[10]. CCK is identified as several different size of the peptide including 4, 8, 33, 39, and 58 amino acid forms. The sulfated carboxy-terminal octapeptide (sCCK-8) is the biologically predominant active form localized in the small intestine, blood and CNS[10]. In the last decade few studies have investigated the relation between CCK and immune cells. Okahata et al[11] demonstrated CCK immunoreactivity in peripheral blood leukocytes. Data from several labs suggest that CCK mediates an increase in intracellular calcium in human peripheral blood mononuclear cells and T lymphocyte cell line[12,13]. sCCK-8 can inhibit the mobility capacity and the mitogen-induced lymphocytic proliferation, but can increase the adherence and the spontaneous proliferation of lymphocytes[14,15]. In addition, sCCK-8 is a negative modulator of several functions of murine peritoneal macrophages and human neutrophils including the production of superoxide anion, phagocytosis and mobility[16-18]. Interestingly, sCCK-8 causes an in vitro inhibition of lipopolysaccharide (LPS)-induced TNF-α production, sCD14 release and mCD14 expression in rat pulmonary interstitial macrophages (PIMs)[19]. Consistently, the production of proinflammatory cytokines including TNF-α, IL-1α, and IL-6 in endotoxin shock (ES) rat was also inhibited by sCCK-8 in vivo[20,21]. These results suggested that sCCK-8 has anti-inflammatory effect to some extent, a new field about the biological action of CCK, which was also confirmed by a morphological observation that sCCK-8 clearly lessened the inflammatory lesions in lung, spleen and liver tissues in ES rat[22].
It is well known that transcriptional factor NF-κB plays a pivotal role in LPS-induced TNF-α gene expression and involved in the inflammatory response[23]. To elucidate the anti-inflammatory mechanism of sCCK-8, we investigated the effects of sCCK-8 on TNF-α mRNA expression and NF-κB activity in the present study.
MATERIALS AND METHODS
Materials
Collagenase IA, LPS (E. Coli 0111: B4), sCCK-8 and proglumide were obtained from Sigma (St. Louis, MO). RPMI-1640 culture medium and TRIzol reagents were obtained from Gibco BRL (Gercy-Pontoise, France). Avian myeloblastosis virus reverse transcriptase and Gel shift assay system were purchased from Promega (Madison, WI). Taq DNA polymerase was obtained from Sangon (Shanghai, China). Coomassie brilliant blue G250 assay kit was purchased from JianCheng Biotechnology (Nanjing, China). An anti-IκBα polyclonal antibody (C-21) was purchased from Santa Cruz (Santa Cruz, CA.) and horseradish peroxidase (HRP)-conjugated IgG was from Zhongshan Biotechnology (Beijing, China). Female, specific pathogen-free Sprague-Dawley rats (weighting 180- 220 g BW) were obtained from Experimental Animal Center of Hebei Province.
Preparation of rat PIMs
PIMs were isolated from perfused rat lungs with a collagenase digestion technique, modified as Wizemann et al[24] described. Alveoli were lavaged 12 to 14 times with 4 °C phosphate-buffered saline (PBS) containing 0.6 mmol·L-1 EDTA (PBS-EDTA) to remove alveolar macrophages. Lung vessles were perfused with 100 mL PBS-EDTA to remove the monocytes and other blood cells. Then the lung tissues were cut into 500 μm slices followed by digestion in 175 U·mL-1 collagenase IA containing 0.1 g·L-1 DNase, and 100 mL·L-1 fetal bovine serum (FBS) in a shaking 37 °C water bath for 60 min. The suspension was then filtered through 30 μm mesh. After washing 2 times (400 × g, 4 °C, 10 min) with PBS, the cells were resuspended in RPMI-1640 medium containing 15 mL·L-1 FBS, 100 U·mL1 penicillin, and 100 μg·mL-1 streptomycin and incubated for 2 h at the conditions of 37 °C and 50 mL·L-1 CO2. Nonadherent cells were removed by gentle washing with warm medium. Remaining adherent cells contained more than 93% PIMs and the contamination by polymorphonuclear leukocytes and alveolar macrophages was less than 7%. PIMs viability was greater than 95%, determined by trypan blue exclusion assay. PIMs (1 × 106) were plated on to culture dishes. After washing 3 times with serum-free medium, PIMs were stimulated with LPS (1 mg·L-1), in the presence or absence of sCCK-8 (10-8-10-6 mol·L-1) or/and the CCK receptor antagonist proglumide (2 mg·L-11) for indicated time.
Semi-quantitative RT-PCR for the detection of TNF-α
To determine whether sCCK-8 affect TNF-α transcription, PIMs were stimulated with LPS in the presence or absence of 10-8-10-6 mol·L-1 sCCK-8 for 3 h. Total cellular RNA was prepared with TRIzol reagents. cDNA was synthesized from 4 μg of the total RNA by extension of random primers with avian myeloblastosis virus reverse transcriptase. PCR of the cDNA was performed in a final volume of 25 μL containing 2 mmol·L-1 MgCl2, 4 units Taq DNA polymerase, and 25 pmol specific primers. Amplification of the house-keeping enzyme glyceraldehydes-3-phosphate dehydrogenase (GADPH) was always involved to serve as control of reaction efficacy. The PCR was performed at the conditions of denaturation for 45 s at 94 °C, annealing for 45 s at 48 °C for TNF-α and 55 °C for GADPH, extension for 45 s at 72 °C, and at the end of 35 cycles, further extension for 5 min at 72 °C. The primers were TNF-α sense, 5'-CCAACAAGGAGGAGAAGT-3' TNF-α antisense, 5'-GTATGAAGTGGCAAATCG-3' (323 bp). The synthesized PCR products were separated by electrophoresis on a 20 g·L-1 agarose gel and analyzed by Gel-Pro analyzer version 3.1 software (Media Cybernetics). The ratio of arbitrary unit (AU, Darea·Ddensity) of target genes over GADPH was used for expressing the relative level of mRNA expression.
Electrophoretic Mobility Shift Assay (EMSA) for NF-κB activity
PIMs were stimulated with LPS in the presence or absence of sCCK-8 for 1 h. Nuclear extracts were prepared essentially as described by Liu et al[25]. Cells were washed with PBS, lysed with five-pellet volume of buffer A containing 10 mmol·L-1 HEPES (pH7.9), 10 mmol·L-1 KCl, 1.5 mmol·L-1 MgCl2, 0.5 mmol·L-1 DTT, 0.5 mmol·L-1 PMSF, and 5 mL·L-1 NP-40 and centrifuged for 10 min at 1850 × g. Then the pellets were resuspended with three-pellet volume of buffer B (same as buffer A, but without NP-40) and incubated on ice for 10 min. The cells were centrifuged for 15 min at 3000 × g to pellet the nuclei. Nuclear pellets were extracted by gently resuspending the nuclei in two thirth-nuclei pellet volume of buffer C (20 mmol·L-1 HEPES (pH7.9), 10 mmol·L-1 KCl, 1.5 mmol·L-1 MgCl2, 200 mL·L-1 glycerol, 0.2 mmol·L-1 EDTA, 0.5 mmol·L-1 DTT, and 0.5 mmol·L-1 PMSF) and one thirth-nuclei pellet volume of buffer D (same as buffer C but with 400 mmol·L-1 KCl). After the nuclei were shaken in buffer C plus buffer D for 60 min on ice, the supernatants were collected by centrifugation at 15000 × g for 30 min at 4 °C. Protein concentration was determined using Coomassie brilliant blue G250 assay kit. Double-stranded deoxyoligonucleotides containing the NF-κB consensus recognition site (5'-AGTTGAGGGGACTTTCCCAGG-3') were end labeled with [γ-32P] ATP using T4 polynucleotide kinase and purified with ethanol precipitation. The nuclear proteins (10 μg per lane) were incubated with the radiolabeled probe DNA (3.5 pmol, 10 μCi) in the presence of 1 mmol·L-1 MgCl2, 0.5 mmol·L-1 EDTA, 0.5 mmol·L-1 DTT, 50 mmol·L-1 NaCl, 10 mmol·L-1 Tris-HCl (pH7.5), 0.05 μg poly (dI-dC), and 40 mL·L-1 glycerol in a final volume of 10 μL. Binding reactions were then incubated at room temperature for 30 min. The DNA-protein complexes were separated on a 6% nondenaturing polyacrylamide gel. The gel was then dried and visualized by autoradiography at -80 °C.
Western blot analysis for IκBα protein level
PIMs were stimulated with LPS in the presence or absence of sCCK-8 for 30 min. Cytoplasmic protein was extracted using lytic buffer containing 100 mmol·L-1 HEPES, 10 mL·L-1 Triton X-100, 1 mmol·L-1 EDTA, 10 mmol·L-1 DTT, and 1 mmol·L-1 PMSF. The lysate was centrifuged 20000 × g for 15 min at 4 °C after ice bath for 15 min. The supernatant was collected and the protein concentration was determined using Coomassie brilliant blue G250 assay kit. Each sample of 100 mg protein was separated on a SDS-10% polyacrylamide gel and transferred to nitrocellulose membrane. The membrane was sequentially blocked in TBS containing 20 g·L-1 milk and then incubated with 2 μg·mL-1 IκBα antibody C-21 (Santa Cruz) overnight, washed, and further incubated with a goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody. Blocking and secondary antibody incubation each lasted 1 h at 37 °C. After several washes, the membrane was developed with DAB. Semi-quantitative analysis of immunoreactivity were measured by Gel-Pro analyzer software, and the results were expressed as AU (Darea·Ddensity).
Statistical analysis
Data were presented as mean ± SD and compared with ANOVA and least significant difference test using SPSS statistical program. A level of P < 0.05 was considered statistically significant.
RESULTS
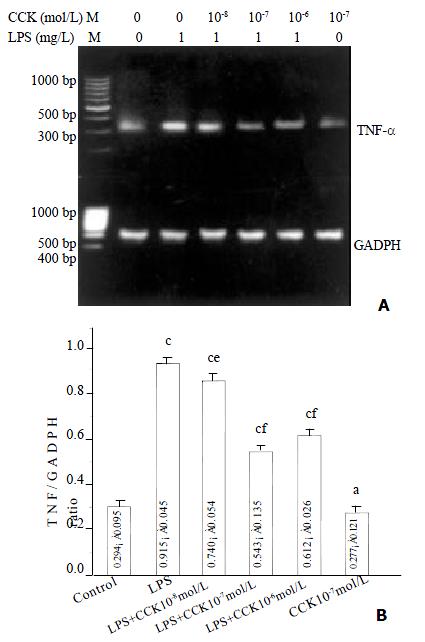
sCCK-8 inhibited LPS-induced TNF-α mRNA expression
Increase in TNF-α mRNA was observed 3 h after stimulating PIMs with 1 mg·L-1 LPS (P < 0.01), and the increase was about 3.1 fold. However in the PIMs co-incubated by LPS and sCCK-8, TNF-α mRNA expression level was obviously lowered in comparison with LPS group (P < 0.05, P < 0.01). The inhibitory rate of 10-8, 10-7 and 10-6 mol·L-1 sCCK-8 was 19%, 41% and 33% respectively. TNF-α mRNA expression level was not affected by sCCK-8 alone (P > 0.05) (Figure 1).
Figure 1 sCCK-8 inhibited LPS-induced TNF-α mRNA expression in PIMs in a dose-dependent manner.
A: Representative pictures of three experiments of RT-PCR. B: Relative level of TNF-α mRNA expression. n = 3, ¯x ± s, aP > 0.05, cP < 0.01 vs control, eP < 0.05, fP < 0.01 vs LPS.
sCCK-8 inhibited LPS-induced NF-κB binding activity
The NF-κB binding activity was significantly higher in PIMs stimulated with 1 mg·L-1 LPS in comparison with unstimulated cells (P < 0.01), and additional treatment with sCCK-8 markedly reduced the binding activity in a dose-dependent manner. sCCK-8 at the concentrations of 10-8, 10-7, and 10-6 mol·L-1 inhibited LPS-induced NF-κB binding activity by 34%, 93% and 100% respectively (P < 0.05, P < 0.01). The effect of sCCK-8 was abrogated by proglumide (P < 0.01). sCCK-8 alone had no effect on the NF-κB binding activity (P > 0.05). The binding specificity was confirmed by using homologous (NF-κB) and nonhomologous (AP-2) oligonucleotides as competitors (Figure 2).
Figure 2 sCCK-8 inhibited LPS-induced NF-κB binding in PIMs in a dose-dependent manner through CCK receptor.
A: Representative autoradiography picture of three experiments of EMSA. Lane 1, negative control; lane 2, positive control; lane 3, 32P-labeled NF-κB Oligo plus unlabeled NF-κB Oligo (competitor); lane 4, 32P-labeled NF-κB Oligo plus unlabeled AP-2 Oligo (noncompetitor); lane 5, control; lane 6, LPS; lane 7, LPS + CCK 10-8 mol·L-1; lane 8, LPS + CCK 10-7 mol·L-1; lane 9, LPS + CCK 10-6 mol·L-1; lane 10, LPS + CCK + proglumide; lane 11, CCK 10-7 mol·L-1. B: Densitometry of EMSA showing decreased NF-κB binding in PIMs treated with sCCK-8. n = 3, ¯x ± s, aP > 0.05, cP < 0.01 vs control; eP < 0.05, fP < 0.01 vs LPS; iP < 0.01 vs LPS + CCK 10-7 mol·L-1.
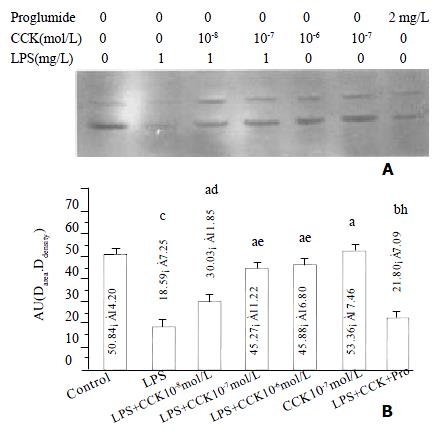
sCCK-8 increased IκBα protein level
The IκBα protein level in PIMs markedly decreased at 30 min of LPS incubation (P < 0.01), sCCK-8 at concentrations of 10-8 - 10-6 mol·L-1 clearly increased IκBα protein level in PIMs exposure to LPS (P < 0.05). The effect was dose dependent and was attenuated by proglumide (P < 0.01). sCCK-8 alone had no effect on the κBa protein level (P > 0.05) (Figure 3).
Figure 3 sCCK-8 increased IκB α protein level at 30 min after LPS stimulation in a dose-dependent manner in PIMs.
A: Representative Western blot of three experiments. B: Normalized total protein of IκBα immunoreactivity. n = 3, ¯x ± s, aP > 0.05, bP < 0.05, cP < 0.01 vs control; dP > 0.05, eP < 0.05, fP < 0.01 vs LPS; hP < 0.05 vs LPS + CCK 10-7 mol·L-1.
DISCUSSION
As an intestinal neuropeptide, sCCK-8 not only protects gastric mucosa against alcohol-induced injury[26-28], but also is a potent protective agent against acute lung injury by LPS[22,29]. It obviously reduced the pulmonary artery hypertention and lessened the inflammatory lesion in lung tissues of ES rats[22,29]. We investigated the effect of sCCK-8 on PIMs since these cells play an important role in the inflammatory response to LPS in the lung. Recently, it was reported that treating rats with LPS resulted in a significant increase in production of reactive oxygen intermediates by PIMs, but not by alveolar macrophages (AM). This treatment also markedly enhanced phagocytosis only in PIMs and caused a significant increase in chemotaxis of PIMs towards C5a. These data demonstrated that PIMs play a role in the inflammatory response of the lung to acute endotoxemia[24]. In this study we showed that incubation of rat PIMs with sCCK-8 caused a decrease in LPS-induced TNF-α mRNA expression level in a dose-dependent manner, which suggested that the inhibitory effect of sCCK-8 on TNF-α production occured at a transcriptional level.
It is well known that transcriptional factor NF-κB plays a pivotal role in LPS-induced TNF-α gene expression[23,30]. The present study indicated that sCCK-8 inhibited NF-κB binding activity in PIMs response to LPS in a dose-dependent manner, which was consistent with our results in the previous in vivo study that sCCK-8 inhibited NF-κB activity in ES rat lung tissues[31]. These results demonstrated that NF-κB might be the upstream mechanism of the inhibitory effect of sCCK-8 on LPS-induced TNF-α mRNA expressions. Because NF-κB activation can lead to enhanced expression of proinflammatory cytokines, chemokines, inflammaroty enzymes such as inducible NO synthase (iNOS) and cycloosygenase (COX-2), adhension molecules and inflammatory receptors[23,30,32,33], modulation of NF-κB activation may provide a direct way of inhibiting inflammatory mediators[34]. Directing drug discovery efforts towards NF-κB activation rather than towards any one of its many target genes could produce a much greater therapeutic benefit by inhibiting expression of the constellation of NF-κB-induced pro-inflammatory genes[35]. Several anti-inflammatory drugs, including corticosteroids, the non-steroidal cyclooxygenase inhibitor sulindac, the benzophenanthridine alkaloid sanguinarine, aspirin and salicylate have all been shown to inhibit NF-κB activation at different stages[35]. Corticosteroids inhibit NF-κB by upregulating the expression of its inhibitor IκB, which displaces DNA-bound NF-κB and restores the latent cytoplasmic IκB-NF-κB complex[35,36]. Sulindac[37], aspirin and salicylate[38] specifically inhibit IκKα kinase activity, and it has also been demonstrated that sanguinarine inhibits NF-κB activation at the level of IκB phosphorylation[39]. In this study, we first demonstrated that the inhibitory effect of sCCK-8 on NF-κB activity was an important signal transduction mechanism of its anti-inflammatory effect. This can also explain the anti-ES role of sCCK-8[22], because NF-κB acts as an important transcriptional factor in the pathogenesis of ES.
Activation of NF-κB is triggered by phosphorylation of an inhibitory subunit, IκB. In unstimulated cells, NF-κB is sequestered in the cytoplasm through interaction with IκB α and IκBα inhibitory proteins. In response to proinflammatory stimuli (e.g. LPS, cytokines, viruses), IκB is first phosphorylated in its N-terminal domain by a large multikinase complex, then polyubiquitinylayted, and finally degraded by the proteasome. The released NF-κB complex translocates into the nucleus where it will initiate gene transcription upon binding its cognate DNA motifs in regulatory segments of TNF-α gene and other target genes involved in inflammatory and immune process[40]. To elucidate the upstream mechanism of sCCK-8 inhibiting NF-κB activity, we further detected whether sCCK-8 affected IκB α protein level with method of Western blot. And the results demonstrated that the decreased IκB α protein level in LPS-stimulated PIMs was elevated by sCCK-8 in a dose-dependent manner. The increase in IκB α protein level could be the result of decreased degradation, increased synthesis, or a combination of these two mechanisms. Because we detected the elevation of IκB α protein level at 30 min of LPS and sCCK-8 incubation, it was not likely that there could be de novo protein synthesis in such short time. So the increase of IκB α protein level was caused by its diminished degradation, suggesting that sCCK-8 inhibited the degradation of IκB α, and subsequently abrogated the translocation to nucleus of NF-κB. However, recently few studies showed that in pancreatic cells, supraphysiological concentrations of CCK (10-7 mol·L-1) induced chemokine expression through the activation of NF-κB[41,42]. CCK (10-7 mol·L-1) also induced the degradation of IκBα in pancreatic cells[41]. So treatment rats with the supraphysiological concentrations of CCK analogue caerulein led to an acute inflammatory response resembling aspects of clinically important disease acute pancteatitis and it had been used to duplicate the experimental model of acute pancreatitis[41,42]. In our study, the same concentrations of CCK-8 (10-7 mol·L-1) showed significant inhibitory effect on NF-κB activation and IκB α degradation in LPS-stimulated PIMs, but had no effect on them in the unstimulated PIMs. This discrepancy may be due to the specificity of the role of CCK in different cells. It is reported that stimulation murine peritoneal macrophages and human neutrophils with CCK led to an inhibition of protein kinase C (PKC) activity[16,18], yet it caused an activation of PKC in rat pancreatic cells[41,42].
Recent data demonstrated the presence of CCK receptors on human monocytes[43] and lymphocytes[44,45]. Our laboratory reported that the pulmonary macrophages expressed CCK receptor detected by in situ hybridization and in situ RT-PCR[46]. In this study, we found that a CCK receptor antagonist proglumide blocked the effects of sCCK-8 on NF-κB binding activity and IκB protein level, indicating that the roles of sCCK-8 were mediated through CCK receptor. Yet the signal transduction mechanisms between CCK receptor and IκB remain to be further determined.
In this study, the concentration of sCCK-8 that showed a distinct effect on LPS-induced TNF-α mRNA expression and NF-κB activity was from 10-8 to 10-6 mol·L-1. However, sCCK-8 alone, at a concentration of 10-7 mol·L-1 had no effect on the above functions of the resting PIMs. Similarly, De la Fuente et al[16] reported that several functions of resting mouse peritoneal macrophages were inhibited by sCCK-8 at the lower concentrations of 10-10-10-8 mol·L-1 but not the higher concentration of 10-6 mol·L-1. They considered that the lower response to higher concentrations of sCCK-8 might be ascribed to a process of cell desen sitization, which is characteristic of sequestration and/or down-regulation of CCK receptor. We reported that LPS up-regulated the expression of CCK receptor[47], so LPS activating PIMs rather than resting PIMs could response to higher concentration of sCCK-8.
In conclusion, our results show that sCCK-8 reduces LPS-induced TNF-α production at transcriptional level by inhibiting NF-κB activity, and the inhibitory effect of NF-κB activity is mediated through the decrease of IκBα degradation, which represents one of the anti-inflammatory mechanisms of sCCK-8.