Published online Aug 15, 2002. doi: 10.3748/wjg.v8.i4.712
Revised: April 10, 2002
Accepted: April 23, 2002
Published online: August 15, 2002
AIM: To study the anti-inflammatory effects of cholecystokinin-octapeptide (CCK-8) on lipopolysaccharide (LPS)-induced endotoxic shock (ES) and further investigate its signal transduction pathways involving p38 mitogen-activated protein kinase (MAPK) and IκB-α.
METHODS: Eighty-four rats were divided randomly into four groups: LPS (8 mg·kg-1, iv) induced ES; CCK-8 (40 μg·kg-1, iv) pretreatment 10 min before LPS (8 mg·kg-1); CCK-8 (40 μg·kg-1, iv) or normal saline (control) groups. The inflammatory changes of lung and spleen, phagocytic function of alveolar macrophage, quantification of inflammatory cells in bronchoalveolar lavage (BAL) were investigated in rats by using hematoxylin and eosin (HE) staining, phagocytosis of Candida albicans and differential cell counting. Nitric oxide (NO) production in serum, lung and spleen was measured with the Griess reaction. The mechanism involving p38 MAPK and IκB-α signal pathways was investigated by Western blot.
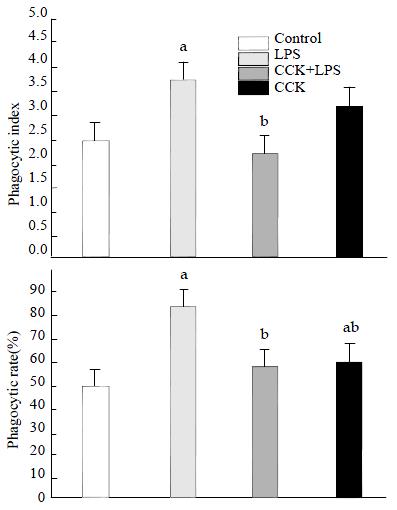
RESULTS: Inflammatory changes of lung and spleen induced by LPS were alleviated by CCK-8, the increase of NO induced by LPS in serum, lung and spleen was significantly inhibited and the neutrophil infiltration in BAL was significantly reduced by CCK-8. The number of neutrophils was (52 ± 10) × 106 cells•L-1 in LPS group, while it decreased to (18 ± 4) × 106 cells•L-1 in CCK-8+LPS (P < 0.01). The phagocytic rate of CCK-8 group increased to (62.49 ± 9.49)%, compared with control group (48.16 ± 14.20)%, P < 0.05. The phagocytosis rate was (85.14 ± 4.64)% in LPS group, which reduced to (59.33 ± 3.14)% in CCK-8+LPS group (P < 0.01). The results of phagocytosis indexes showed similar changes. CCK-8 may play an important role in increasing the expression of p38 MAPK and decreasing the degradation of IκB-α in lung and spleen of ES rats.
CONCLUSION: CCK-8 can result in anti-inflammatory effects, which may be related to activation of p38 MAPK and inhibition on the degradation of IκB-α.
- Citation: Meng AH, Ling YL, Zhang XP, Zhang JL. Anti-inflammatory effect of cholecystokinin and its signal transduction mechanism in endotoxic shock rat. World J Gastroenterol 2002; 8(4): 712-717
- URL: https://www.wjgnet.com/1007-9327/full/v8/i4/712.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i4.712
Lipopolysaccharide (LPS), a main component of Gram-negative bacterial endotoxin[1], is the leading cause of sepsis or endotoxic shock (ES), and when administered experimentally to animals, it results in the same inflammatory response mimically. Physical stress such as infection can stimulate proinflammatory cytokine production and release. The overproduction of these cytokines has been postulated to contribute to the development of tissue injury[2]. The pathogenesis of inflammatory sepsis is also linked to the overproduction of nitric oxide (NO), a potentially toxic molecule, being possibly responsible in part for the cytotoxicity of the inflammatory process[3]. Nuclear factor (NF)-κB is a heterodimeric protein complex containing two members of the rel family of transcription factors, p50 and p65. At rest, the heterodimeric NF-κB complex is located in the cytoplasm bound to an inhibitory factor, I-κB. Upon stimulation, IκB-α is phosphorylated and proteolytically degraded or processed by proteasomes and other proteases. Free NF-κB then translocates into the nucleus where it binds to various gene promoter regions controlling the expression of various pro-inflammatory and proliferative agents[4]. NO augments the activation of NF-κB in macrophages and, therefore, may play a role in producing a positive cycle of inflammation[5]. One of the earliest responses to LPS is activation of the mitogen-activated protein kinase (MAPK) homolog p38. The p38 MAPK is involved in intracellular signals that regulate a variety of cellular responses during inflammation[6]. A slightly later cellular response to LPS is the activation of NF-κB, which does not require p38 kinase activity[7].
Cholecystokinin (CCK), a component from the gastrin-CCK family, first isolated from hog intestine, shows a widespread distribution in different organs and tissues. The sulfated carboxy-terminal octapeptide (CCK-8), isolated from the central nervous system and digestive tract, is the predominant active form. CCK-8 possessed both excitatory and inhibitory action on contractile activity of different regions of stomach in guinea pigs[8]. CCK-8 could antagonize the elimination of morphine on the potentiations of ACh to duodenal activities[9]. Besides the effects on the digestive tract, other biological actions of CCK-8 have been observed, for instance appetite inhibition and so on[10,11]. In the spleen, CCK-8 is formed in high abundance in the white pulp where it appears to surround cell clusters. It seems that CCK-8 increases the secretion of immunoglobulins in vivo[12], whereas it inhibits Molt-4 lymphoblast proliferation[13] and modulates mitogen-induced lymphoproliferation and intracellular calcium mobilization in vitro[14,15]. CCK-8, a chemoattractant for human monocytes and rat macrophages[16], enhanceing human eosinophil chemotaxis induced by PAF and LTB4 in allergic patient[17] is a negative modulator of several murine macrophage and human neutrophil functions[18,19].
Our previous in vivo and in vitro study demonstrated that CCK-8 could protect animals from ES[20,21], which was related to its inhibitory effect on the overproduction of proinflammatory cytokines[22] and on the transcription of TNF-α[23]. In the present study, the effects of CCK-8 on NO production, inflammatory changes of lung and spleen induced by LPS and phagocytic function of rat alveolus macrophage and further on the p38 MAPK and IκB-α expression in lung and spleen were investigated.
CCK-8 (sulfated), LPS (E. coli LPS, serotype 0111:B4), leupeptin, pepstatin A, Triton X-100 and p38 monoclonal antibody were all purchased from Sigma, RPMI-1640 from Gibcobrl, aprotinin from Boehringer, IκB-α polyclonal antibody from Santa Cruz. All other reagents used were of analytic grade.
Animal preparation[22] Sprague-Dawley rats (150-200 g BW, Experimental Animal Center of Hebei Province) were randomly assigned to four groups injected different agents via tail vein. For group receiving LPS, a bolus dose (8 mg/kg) of LPS was injected into the tail vein. For group of CCK-8+LPS, a bolus dose (40 μg/kg) of CCK-8 was administered 10 min before the injection of LPS. Negative control animals received saline. CCK-8 (40 μg/kg) was also administered alone in the other group.
Sample collection Animals were sacrificed at 2 h, 6 h or 12 h, spleen and lung were rapidly excised and rinsed of blood, and blood was taken and centrifuged to collect serum. The samples were stored at -80 °C for analysis of NO content. Additional groups of animals were sacrificed at 30 min and their lungs and spleens excised were analyzed for p38 MAPK and IκB-α expression.
Histological examination Rats were treated as described in animal preparation and sacrificed at 2 h, 6 h or 12 h after LPS administration. Lung and spleen were sliced into pieces and preserved in 10% formalin. Tissue samples were embedded in paraffin, cut into 5-μm sections, and then assessed by routine staining with hematoxylin and eosin (HE) and examined by light microscopy.
Nitrate/nitrite analysis The samples (serum, spleen, lung) collected 2 h, 6 h or 12 h were analyzed. Tissues were homogenized with PBS (4 °C, pH7.2, 100 mg tissue/mL) and centrifuged at 12000 rpm, 10 min. Supernatants and serum were assayed NO content based on the Griess reaction, which consists of measurement of stable end breakdown products of NO such as nitrite that are considered to be reliable markers for NO formation. The nitrite was converted to a deep purple compound and absorbance was read at 550 nm, and nitrite concentration was determined using NaNO2 standards.
Phagocytic function Two hours after injection of LPS, bronchus alveolus lavage fluid (BALF) was obtained and macrophages were isolated from BALF by centrifugation (1500 rpm, 10 min)[6]. Being washed with normal saline (NS), the cells were resuspended in RPMI-1640, adjusted to 106/mL and incubated for 2 h. The adherent monolayer was washed with RPMI-1640 and then aliquots of Candida albicans (2 × 107 cells/mL medium), previously in water bath (100 °C) for 30 min were added. After 60 min of incubation, the plates were washed with NS, fixed and stained and the number of yeasts ingested per 100 macrophages was counted. Phagocytosis rate and phagocytosis index were calculated according to the following equation:
Phagocytosis rate (%) = (macrophages with yeasts ingested/total macrophages)%
Phagocytosis index = number of Candida albicans ingested/number of macrophages with Candida albicans ingested
Quantification of inflammatory cells Neutrophil influx was determined in the BALF samples obtained 2 h by differential cell counting. Total whole cell counts were obtained in BALF. The cells were smeared on slides and stained with Wrights-Giemsa stain. Three differential counts on 200 cells per slide were performed and the percentage of macrophages/monocytes, lymphocytes and neutrophils were determined.
Western blot Lung and spleen were excised 30 min after injection of LPS. Tissues were homogenized in a buffer containing 50 mM Tris (pH7.5), 150 mM NaCl, 1% TritonX-100, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM NaF, 1 mM sodium vanadate and a 40 μg/mL protease inhibitor cocktail and centrifuged at 18000 rpm, 4 °C, 10 min. Supernatants were fractionated on 12% SDS-polyacrylamide gels, transferred to poly (vinylidene difluoride) membranes, and incubated with phospho-specific anti-p38 MAPK or IκB-α polyclonal antibody. Being washed three times in T-PBS, membranes were incubated in horseradish peroxidase-linked secondary antibody for 1 h at room temperature. Membranes were again washed three times with T-PBS and stained with diaminobenzidine (DAB).
Data were reported as ¯x ± s. Statistical differences between values from different groups were determined by one way ANOVA and Newman-Keuls q test. Significance was set at P < 0.05.
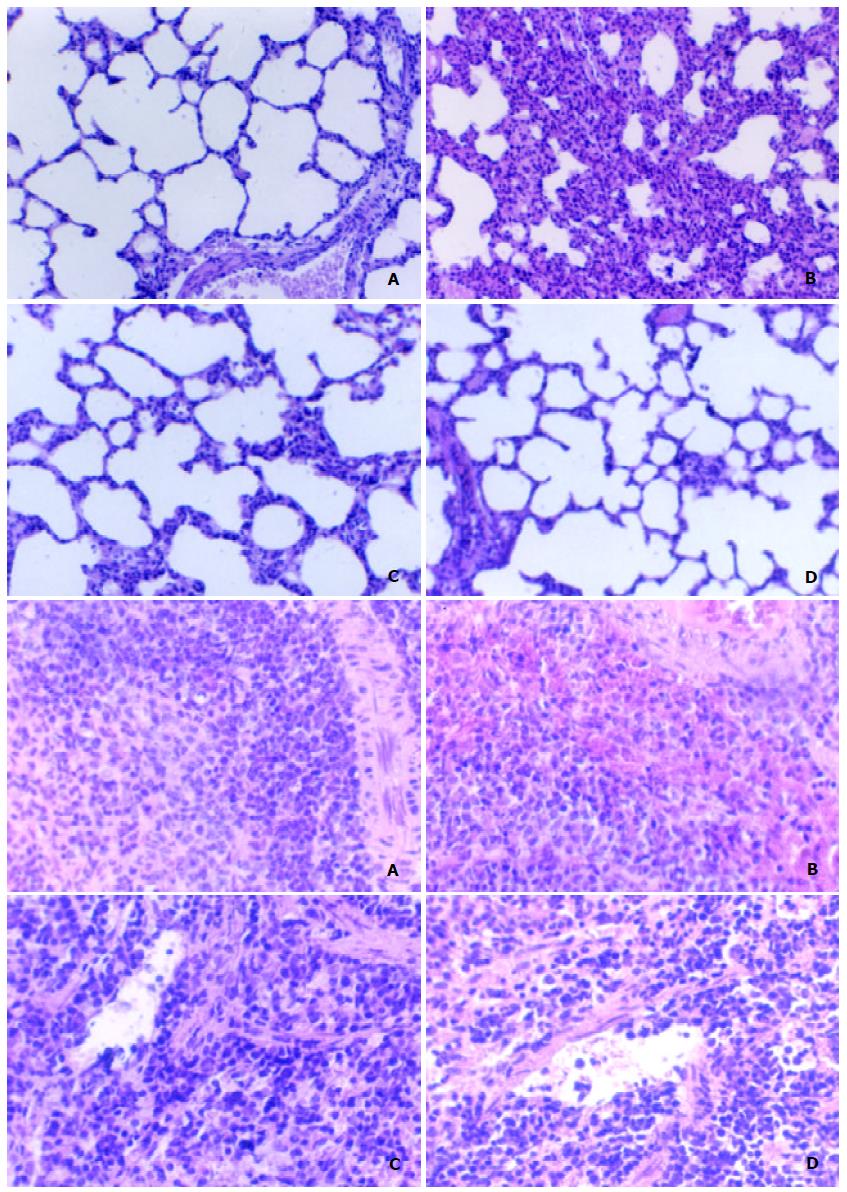
Lung and spleen were harvested at 2 h, 6 h or 12 h. There were structural injury in LPS group and alleviated changes in CCK-8+LPS group at 2 h, while more significant changes at 6 h and 12 h. The lung of LPS-stimulated rats demonstrated widened septa of alveoli, diffuse infiltration and migration of acute inflammatory cells (PMN) accompanied by atrophied or disappeared alveoli, and slight alveolar atelectasis or emphysema. While in CCK-8+LPS group, the inflammatory changes were evident, but to a lesser extent. The spleen of LPS-stimulated rats showed hyperaemia in spleen sinusoids with concentrated red blood cells (RBC) and PMN, while it changes to a lesser extent in CCK-8+LPS group. There was not much difference between CCK-8 group and control group (Figure 1).
Studies of LPS-induced NO production in spleen, lung and serum showed increase in either nitrate or nitrite levels at 2 h, 6 h or 12 h after LPS administration. Significant increase was observed at 6 h, compared with that in control animals. CCK-8 produced inhibitory effect on increase of NO content induced by LPS (Table 1).
| Group | 2 h | 6 h | 12 h |
| Serum (μmol•L-1) | |||
| Control | 36.8 ± 2.2 | 39.2 ± 3.2 | 33.8 ± 4.1 |
| LPS | 78.2 ± 7.4b | 250.9 ± 19.6b | 302.9 ± 41.2b |
| CCK-8 + LPS | 48.5 ± 5.3d | 124.8 ± 4.9bd | |
| CCK-8 | 37.3 ± 2.3 | 32.4 ± 6.6 | |
| Lung (μmol•g-1) | |||
| Control | 48.9 ± 7.6 | 47.2 ± 4.7 | 51.2 ± 6.1 |
| LPS | 117.5 ± 6.7b | 140.6 ± 24.1b | 186.6 ± 8.3b |
| CCK-8 + LPS | 81.9 ± 10.8bd | 114.8 ± 8.3bd | |
| CCK-8 | 52.6 ± 5.6 | 44.0 ± 2.3 | |
| Spleen (μmol•g-1) | |||
| Control | 53.9 ± 5.6 | 48.5 ± 9.0 | 49.8 ± 6.1 |
| LPS | 71.3 ± 8.9b | 74.7 ± 4.2a | 63.5 ± 3.8a |
| CCK-8 + LPS | 59.6 ± 3.3c | 46.5 ± 11.0d | |
| CCK-8 | 45.0 ± 2.9 | 42.7 ± 2.3 |
Phagocytosis of alveolar macrophages isolated from BALF 2 h after LPS administration was increased significantly, the phagocytosis index increased from 2.43 ± 0.71 (Control) to 3.80 ± 0.60 (P < 0.05). CCK-8 showed an inhibitory effect on the LPS-induced increase of Candida albicans phagocytosis by macrophage. The phagocytosis index in CCK-8 + LPS group reduced to 2.21 ± 0.14, P < 0.05. While the phagocytosis rate of CCK-8 group was (62.49 ± 9.49)%, which was higher than control group (48.16 ± 14.20)%, P < 0.05 (Figure 2).
Compared with Control, significant increase of BAL neutrophils was observed in LPS group, The number increased from (1 ± 0.2) × 106 cells·L-1 to (52 ± 10) × 106 cells·L-1, P < 0.01. It decreased to (18 ± 4) × 106 cells·L-1, P < 0.01, in CCK-8+LPS group compared with LPS group.
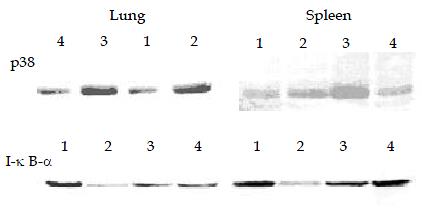
Significant phosphorylation of p38 MAPK was observed in lung and spleen of endotoxic rats 30 min post LPS administration. CCK-8 can enhance LPS-induced phosphorylation of p38 MAPK significantly. Phosphorylation of p38 MAPK was also observed in animals receiving normal saline or CCK-8. The degradation of IκB-α in lung and spleen following LPS administration was observed, while the procedure of pre-administration of CCK-8 could reduce its degradation (Figure 3).
Some studies examined ex vivo measures of immune function stimulated by LPS or CCK-8. While ex vivo determinations of immune function are useful, and yield important information concerning the status of the immune system, there is the concern that ex vivo stimulation of immune cells may not be naturalistic and consequently be of limited value when immunocompetence is assessed in a whole organism. Thus another approach which has been used to assess immunological status in laboratory animals, is to examine their ability to respond to an in vivo challenge with bacterial LPS[24]. Macrophages are the sources of proinflammatory cytokines[25]. Inflammatory mediators like cytokines, NO and reactive oxygen species are released by activated macrophages from various sources, including spleen and lung. NO is particularly important as a vasodilator but also participates in immunologic reactions, including the ability of macrophages to kill tumor cells and bacteria[26]. Some of the deleterious effects of LPS on organ function have been attributed to NO[27]. CCK-8 exerts an inhibitory effect on the overproduction of proinflammatory cytokines induced by LPS, which suggested its functions of anti-inflammatory effect[22]. In addition, CCK-8 inhibits LPS-induced increase of NO, which may be related to its inhibition on inducible NO synthase[28]. In contrast, our data do not support the idea that CCK-8 may be mediating the anti-inflammatory effects by the production of NO, to which anti-inflammatory effects also have been described[29].
Members of the MAPK cascade are considered to play key roles in signal transduction pathways activated by a wide range of stimuli. The three best characterized members of this growing family of serine/threonine kinases are extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK) and p38. Active MAPK are responsible for the phosphorylation of a variety of effector proteins including several transcription factors[30]. P38 may help reduce organ destruction while inhibition of p38 during induction of cerulein pancreatitis leads to the occurrence of acinar necrosis[31]. Recent evidence from other systems indicates that p38 activation can indeed be protective. Thus, p38 is reportedly important for protection observed after ischemic precondition in myocardial cells and it is evidenced that p38 may exert protective effects via its substrate MAPKAPK2 (MAPK activated protein kinase 2)[32,33]. Activated p38 inhibited iNOS induction, which may be due to the ability of p38 to inhibit LPS-induced JNK activation[34,35]. The anti-inflammatory effects of CCK-8 seem to involve the p38 MAPK pathway. The mechanism by which CCK-8 modulates the p38 MAPK remains unclear at this time. Sodium salicylate suppress TNF-α production, activates p38 MAPK and inhibits both ERK1/ERK2 and SAPK/JNK MAPKs in LPS-stimulated macrophages. Additionally, it has been shown that p38 activation is required for IκB-mediated inhibition of NF-κB[36]. It was reported that LPS-induced production of TNF-α was regulated mainly, but not exclusively, through the p38 MAPK pathway[37-40]. Other signal pathway may be involved. Induction of tolerance by sublethal hemorrhage (SLH) is dependent of p38 MAPK activation and this intracellular signal may be a necessary step in the initiation of the cellular reprogramming associated with tolerance. The normal activation of p38 MAPK is preserved in response to a "second insult" with LPS despite an attenuation in TNF production by tolerant cell[41].
NF-κ plays an important role in physiologic and pathologic conditions as an inducible nuclear factor. NF-κB/Rel have been implicated in the inflammatory response. Degradation of IκB-α proteins frees NF-κB proteins, which then translocate into the nucleus, where they activate transcription[42]. Another study from our laboratory showed CCK-8 inhibited the increased activity of NF-κB induced by LPS in lung of rats, which may be related to its anti-inflammatory effect.
CCK-8 inhibits phagocytic function of alveolar macrophages isolated 2 h after LPS administration. In vivo, this would prevent the excess accumulation of phagocytic cells in the inflamed area, and thereby decrease the phagocytic process. This may be related to its anti-inflammatory effect. Interestingly, CCK-8 itself increases the phagocytic process compared with control. The result indicated that CCK-8 may play different immunoregulatory role in different conditions[43]. The development of tissue damage in shock is closely associated with the release of an ever-increasing number of mediators and acculmulation of neutrophils at the sites of infection or injury[44]. CCK-8 alleviated the accumulation of neutrophils in BALF, which may be related to its protecting effect on lung of ES rats.
The results of the present study show that administration of CCK-8 prevents inflammatory changes in lung and spleen of rats and attenuates increase of NO and inflammatory cells induced by LPS. Moreover, CCK-8 inhibits LPS-induced increase of macrophage phagocytic function while itself increases phagocytic function. CCK-8 enhances the expression of p38 MAPK while reduces the degradation of IκB-α induced by LPS, which may be involved in the signal transduction pathway.
Edited by Zhao P
| 1. | Fan K. Regulatory effects of lipopolysaccharide in murine macrophage proliferation. World J Gastroenterol. 1998;4:137-139. [PubMed] |
| 2. | Molina PE, Abumrad NN. Differential effects of hemorrhage and LPS on tissue TNF-alpha, IL-1 and associate neuro-hormonal and opioid alterations. Life Sci. 2000;66:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Zhang GL, Wang YH, Teng HL, Lin ZB. Effects of aminoguanidine on nitric oxide production induced by inflammatory cytokines and endotoxin in cultured rat hepatocytes. World J Gastroenterol. 2001;7:331-334. [PubMed] |
| 4. | Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7-17. [PubMed] |
| 5. | Kang JL, Lee K, Castranova V. Nitric oxide up-regulates DNA-binding activity of nuclear factor-kappaB in macrophages stimulated with silica and inflammatory stimulants. Mol Cell Biochem. 2000;215:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Ohashi N, Matsumori A, Furukawa Y, Ono K, Okada M, Iwasaki A, Miyamoto T, Nakano A, Sasayama S. Role of p38 mitogen-activated protein kinase in neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol. 2000;20:2521-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Garrett TA, Rosser MF, Raetz CR. Signal transduction triggered by lipid A-like molecules in 70Z/3 pre-B lymphocyte tumor cells. Biochim Biophys Acta. 1999;1437:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Li W, Zheng TZ, Qu SY. Effect of cholecystokinin and secretin on contractile activity of isolated gastric muscle strips in guinea pigs. World J Gastroenterol. 2000;6:93-95. [PubMed] |
| 9. | Xu MY, Lu HM, Wang SZ, Shi WY, Wang XC, Yang DX, Yang CX, Yang LZ. Effect of devazepide reversed antagonism of CCK-8 against morphine on electrical and mechanical activities of rat duodenum in vitro. World J Gastroenterol. 1998;4:524-526. [PubMed] |
| 10. | De la Fuente M, Carrasco M, Del Rio M, Hernanz A. Modulation of murine lymphocyte functions by sulfated cholecystokinin octapeptide. Neuropeptides. 1998;32:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Du YP, Zhang YP, Wang SC, Shi J, Wu SH. Function and regulation of cholecystokinin octapeptide, beta-endorphin and gastrin in anorexic infantile rats treated with ErBao Granules. World J Gastroenterol. 2001;7:275-280. [PubMed] |
| 12. | Alverdy J, Stern E, Poticha S, Baunoch D, Adrian T. Cholecystokinin modulates mucosal immunoglobulin A function. Surgery. 1997;122:386-92; discussion 392-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Tang SC, Braunsteiner H, Wiedermann CJ. Regulation of human T lymphoblast growth by sensory neuropeptides: augmentation of cholecystokinin-induced inhibition of Molt-4 proliferation by somatostatin and vasoactive intestinal peptide in vitro. Immunol Lett. 1992;34:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Elitsur Y, Luk GD. The inhibition effect of cholecystokinin in human colonic lamina propria lymphocyte proliferation, and reversal by the cholecystokinin receptor antagonist L-364718. Neuropeptides. 1991;20:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | McMillen MA, Ferrara A, Adrian TE, Margolis DS, Schaefer HC, Zucker KA. Cholecystokinin effect on human lymphocyte ionized calcium and mitogenesis. J Surg Res. 1995;58:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Sacerdote P, Ruff MR, Pert CB. Cholecystokinin and the immune system: receptor-mediated chemotaxis of human and rat monocytes. Peptides. 1988;9 Suppl 1:29-34. [PubMed] |
| 17. | Numao T, Agrawal DK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol. 1992;149:3309-3315. [PubMed] |
| 18. | De la Fuente M, Campos M, Del Rio M, Hernanz A. Inhibition of murine peritoneal macrophage functions by sulfated cholecystokinin octapeptide. Regul Pept. 1995;55:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Carrasco M, Del Rio M, Hernanz A, De la Fuente M. Inhibition of human neutrophil functions by sulfated and nonsulfated cholecystokinin octapeptides. Peptides. 1997;18:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Ling YL, Huang SS, Wang LF, Zhang JL, Wan M, Hao RL. [Cholecystokinin-octapeptide (CCK-8) reverses experimental endotoxin shock]. Shengli Xuebao. 1996;48:390-394. [PubMed] |
| 21. | Meng AH, Ling YL, Wang DH, Gu ZY, Li SJ, Zhu TN. [Cholecystokinin-octapeptide alleviates tumor necrosis factor-alpha induced changes in rabbit pulmonary arterial reactivity and injuries of endothelium in vitro]. Shengli Xuebao. 2000;52:502-506. [PubMed] |
| 22. | Ling YL, Meng AH, Zhao XY, Shan BE, Zhang JL, Zhang XP. Effect of cholecystokinin on cytokines during endotoxic shock in rats. World J Gastroenterol. 2001;7:667-671. [PubMed] |
| 23. | Meng AH, Ling YL, Zhang XP, Zhao XY, Zhang JL. CCK-8 inhibits expression of TNF-alpha in the spleen of endotoxic shock rats and signal transduction mechanism of p38 MAPK. World J Gastroenterol. 2002;8:139-143. [PubMed] |
| 24. | Connor TJ, Kelly JP, McGee M, Leonard BE. Methylenedioxymethamphetamine (MDMA; Ecstasy) suppresses IL-1beta and TNF-alpha secretion following an in vivo lipopolysaccharide challenge. Life Sci. 2000;67:1601-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Wu XN. Current concept of pathogenesis of severe acute pancreatitis. World J Gastroenterol. 2000;6:32-36. [PubMed] |
| 26. | Wu GH, Zhang YW, Wu ZH. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J Gastroenterol. 2001;7:357-362. [PubMed] |
| 27. | Mailman D, Guntuku S, Bhuiyan MB, Murad F. Organ sites of lipopolysaccharide-induced nitric oxide production in the anesthetized rat. Nitric Oxide. 2001;5:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Meng AH, Ling YL, Wang DH, Gu ZY, Li SJ, Zhu TN. [Role of nitric oxide in cholecystokinin octapeptide alleviation of tumor necrosis factor alpha induced changes in rabbit pulmonary arterial reactivity]. Shengli Xuebao. 2001;53:478-482. [PubMed] |
| 29. | Dérijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1287] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 30. | Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143-180. [PubMed] |
| 31. | Fleischer F, Dabew R, Göke B, Wagner AC. Stress kinase inhibition modulates acute experimental pancreatitis. World J Gastroenterol. 2001;7:259-265. [PubMed] |
| 32. | Weinbrenner C, Liu GS, Cohen MV, Downey JM. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. J Mol Cell Cardiol. 1997;29:2383-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Nakano A, Baines CP, Kim SO, Pelech SL, Downey JM, Cohen MV, Critz SD. Ischemic preconditioning activates MAPKAPK2 in the isolated rabbit heart: evidence for involvement of p38 MAPK. Circ Res. 2000;86:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Chan ED, Morris KR, Belisle JT, Hill P, Remigio LK, Brennan PJ, Riches DW. Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-kappaB signaling pathways. Infect Immun. 2001;69:2001-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Chan ED, Riches DW. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 (mapk) in a mouse macrophage cell line. Am J Physiol Cell Physiol. 2001;280:C441-C450. [PubMed] |
| 36. | Vittimberga FJ, McDade TP, Perugini RA, Callery MP. Sodium salicylate inhibits macrophage TNF-alpha production and alters MAPK activation. J Surg Res. 1999;84:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Geppert TD, Whitehurst CE, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994;1:93-103. [PubMed] |
| 38. | Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther. 1996;279:1453-1461. [PubMed] |
| 39. | Wysk M, Yang DD, Lu HT, Flavell RA, Davis RJ. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc Natl Acad Sci USA. 1999;96:3763-3768. [PubMed] |
| 40. | Haddad EB, Birrell M, McCluskie K, Ling A, Webber SE, Foster ML, Belvisi MG. Role of p38 MAP kinase in LPS-induced airway inflammation in the rat. Br J Pharmacol. 2001;132:1715-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Mendez C, Jaffray C, Wong V, Salhab KF, Kramer AA, Carey LC, Norman JG. Involvement of p38 mitogen-activated protein kinase in the induction of tolerance to hemorrhagic and endotoxic shock. J Surg Res. 2000;91:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Guo SP, Wang WL, Zhai YQ, Zhao YL. Expression of nuclear factor-kappa B in hepatocellular carcinoma and its relation with the X protein of hepatitis B virus. World J Gastroenterol. 2001;7:340-344. [PubMed] |
| 43. | Carrasco M, Hernanz A, De La Fuente M. Effect of cholecystokinin and gastrin on human peripheral blood lymphocyte functions, implication of cyclic AMP and interleukin 2. Regul Pept. 1997;70:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J Gastroenterol. 2001;7:128-130. [PubMed] |