Published online Aug 15, 2002. doi: 10.3748/wjg.v8.i4.658
Revised: February 4, 2002
Accepted: February 7, 2002
Published online: August 15, 2002
AIM: To evaluate the role and limitation of fast multiplanar spoiled gradient-recalled (FMPSPGR) MR dynamic contrast scanning in the follow-up of patients with HCC treated by transarterial chemoembolization (TACE).
METHODS: Twenty-two patients with 24 HCC lesions confirmed by biopsy or surgical resection underwent MR imaging in 4-9wks after TACE with a superconducting 1.5 T MR scanner, including SE T1WI, T2WI and FMPSPGR dynamic contrast scanning. The signal intensities of all lesions on SE T1WI, T2WI and the enhancement patterns on FMPSPGR dynamic contrast scanning were observed, and the comparison was made between MRI findings and pathological results in all the cases.
ESULTS: Of the 24 lesions, the signal intensities were various on SE T1WI and T2WI. On T1WI, 13 lesions appeared as hyperintense, 4 lesions were isointense and the other 7 lesions were hypointensese. Histologically, hyperintense lesions showed on T1WI were viable tumor or hemorrhage; isointensities were coagulative necrosis or inflammatory infiltration; hypointensities were tumor, liquified necrosis, coagulative necrosis or inflammatory infiltration. On T2WI, 15 lesions appeared as hyperintense, 3 lesions were isointense and the other 6 lesions were hypointensese. Hyperintense lesions showed on T2WI were residuals of viable tumor, hemorrhage, liquefied necrosis or inflammatory infiltration; isointense lesions were residuals of viable tumor or inflammatory infiltration; hypointense lesions were coagulative necrosis. On FMPSPGR dynamic contrast scanning, 18 of the 24 lesions enhanced on early-phase dynamic scanning corresponding to residuals of viable tumor and the other 6 lesions had no enhancement at this phase because complete necrosis were seen in the histologic examination. On delayed-phase dynamic scanning, 6 lesions had permanent enhancement appeared as inhomogeneous hyperintensity and both residuals of viable tumor and inflammatory infiltration were found by histologic examination. 18 lesions were hypointense at this phase and 8 of them coexisted with peripheral ring-like enhancement of the lesions resulting from viable tumors or inflammatory infiltration.
CONCLUSION: FMPSPGR MR dynamic contrast scanning can reflect the pathologic changes of HCC treated by TACE. Especially, early-phase dynamic scanning can evaluate accurately residuals of viable tumor and necrosis in HCC lesions. FMPSPGR dynamic contrast scanning is useful in the follow-up of patients with HCC treated by TACE combined with SE T1WI and T2WI, but it is difficult to differentiate peripheral viable tumors from inflammatory infiltration.
- Citation: Yan FH, Zhou KR, Cheng JM, Wang JH, Yan ZP, Da RR, Fan J, Ji Y. Role and limitation of FMPSPGR dynamic contrast scanning in the follow-up of patients with hepatocellular carcinoma treated by TACE. World J Gastroenterol 2002; 8(4): 658-662
- URL: https://www.wjgnet.com/1007-9327/full/v8/i4/658.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i4.658
Hepatocellular carcinoma (HCC) is a malignancy prevalent in the Asia countries. Surgical resection is the first choice for the treatment, but this option is not always possible because of coexisting severe cirrhosis, multiple lesions and other conditions not suitable for surgery. In case of unresectable HCC, transarterial chemoembolization (TACE) has been one of the widely used and effective treatment methods and demonstrated great potential in improving survival time, especially, it has been proved to be more effective in combination with percutaneous ethanol injection, Chinese traditional medicine or laser thermal ablation, etc.[1-31]. Since TACE is difficult to kill the tumor cells for once completely, the treatment efficacy of TACE was influenced by many factors, such as the size of tumors, blood supply and the ultra- selectivity of the catheter etc. It is very important to assess objectively the viability and necrosis of the tumors after TACE in HCC, and to take further treatment to improve the general therapeutic effects and the survival rate. Magnetic resonance imaging (MRI) has been a useful modality in the diagnosis of HCC, especially fast multiplannar spoiled gradient recalled (FMPSPGR) sequence dynamic contrast scanning could reflect sufficiently the blood supply of HCC[32-39]. The therapeutic effects of TACE in HCC were evaluated with MRI in this study, and the role and limitation of FMPSPGR dynamic contrast scanning in the follow-up of patients with HCC treated by TACE were discussed.
Twenty-two cases with massive or nodular types of HCC were collected from Sept. 1997 to May 1999. All cases were confirmed by biopsy (n = 19) or surgical resection after TACE (n = 3).
Transarterial chemoembolization was performed by selectively introducing a catheter into the hepatic artery and injecting antitumor agents (5-FU 1000 mg, Cisplatin 80 mg or Biaoroubixing 60-70 mg). Subsequently, the peripheral embolization of the tumors was done with 38% ultrafluid iodized oil (Lipiodol) (10-25 mL) mixed with Mitomycin 16-20 mg, then the central embolization of the tumors was done with 3-5 strips of gelatine sponge (0.1-0.2 cm × 1 cm).
MRI was performed in 4-9 wk after TACE with a superconducting 1.5 T MR scanner (GE Medical Systems Milwaukee, WI), including T1WI (TR/TE = 500-700 ms/14-16 ms) and T2WI (TR/TE = 2000-4000 ms/30-90 ms) and FMPSPGR dynamic contrast MRI (Matrix 256 × 128, thickness 7 mm, gap 3 mm, TR/TE Flip Angle = 100-150 ms/1.6-4.6 ms/60o - 90o. Three to four repeated acquisitions were obtained at 25 s, 60 s, 90 s and 180 s respectively following power injection of 15-20 mL (0.15 mmol/kg) of Gd-DTPA (gadopentetic dimeglumine, Magnevist, Shering Pharmaceutical Ltd.) via antecubital vein.
Nineteen cases underwent biopsy with the CT guidance. The other 3 cases underwent surgical resection after TACE. The MRI images of all cases were read and analyzed by 2 experienced radiologists. The comparison of MRI images between before and after TACE was done in 6 cases. The comparison was also done between MRI findings and pathological results in all cases.
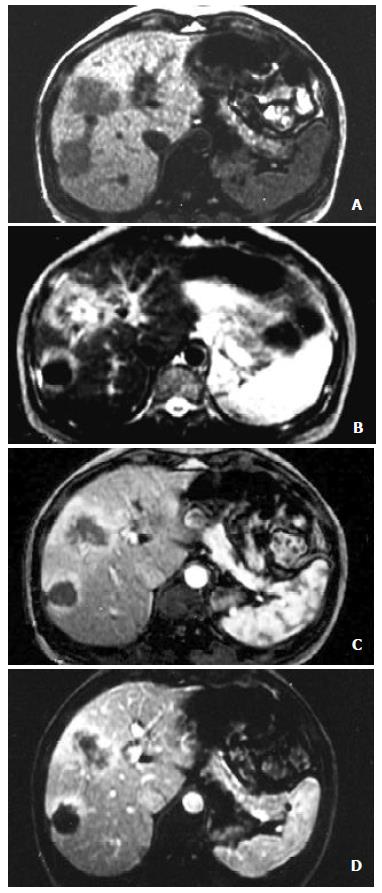
A total of 24 lesions were identified in the 22 cases of HCC. The size of the lesions ranged 3.9-8.2 cm in diameter, with average 5.3 cm (Figure 1).
Results were showed in Table 1.
| SE sequence | signal intensity | ||||
| hyperintensity | isointensity | hypointensity | |||
| homogeneity | inhomogeneity | homogeneity | inhomogeneity | ||
| T1WI | 9 | 4 | 4 | 2 | 5 |
| T2WI | 3 | 12 | 3 | 3 | 3 |
Eighteen lesions were enhanced at the dynamic early phase, in which one of the lesions revealed homogeneous hyperintensity, and the other 17 were inhomogeneous hyperintense. 6 lesions showed no enhancement on this phase. At the late phase of contrast scanning, 6 lesions were still inhomogeneous hyperintense, 18 hypointense and 10 homogeneous hypointensity, and peripheral ring-like enhancement of the tumors were seen in 8 lesions.
Results were showed at Table 2 and Table 3
| SE sequence | signal intensity | Pathological changes |
| hyperintensity | viable tumors, hemorrhage | |
| isointensity | viable tumors, coagulative necrosis, inflammatory infiltration | |
| T1WI | hypointensity | viable tumors, coagulative necrosis, liquefied necrosis, inflammatory infiltration |
| hyperintensity | viable tumors, hemorrhage, liquefied necrosis, inflammatory infiltration | |
| T2WI | isointensity | viable tumors, inflammatory infiltration |
| hypointensity | coagulative necrosis |
| FMPSPGR sequence | enhancement | pathological changes |
| enhanced early phase | enhanced area | viable tumors |
| no enhanced area | necrosis | |
| enhanced area | viable tumors, inflammatory infiltration | |
| enhanced late phase | no enhanced area | viable tumors, necrosis |
| peripheral ring-like | viable tumors, inflammatory infiltration | |
| enhancement |
The MRI findings corresponding to the results of pathology showed that complete necrosis was seen in 6 lesions and various degrees of necrosis coexisted with viable tumors were seen in 17 lesions, except one. Peripheral ring-like enhancement of tumors seen on the FMPSPGR dynamic contrast late phase scanning, could be difficult to be differentiated viable tumors from inflammatory infiltration because of the limitation of bioptic spots.
TACE has been applied in unresectable HCC as an efficient therapy to improve the survival rate and also as a preoperative modality in some HCC patients to make the tumors diminution and then underwent surgical resection[5-31]. Since TACE is difficult to kill all the tumor cells for once completely, so it is generally used repeatedly. It is needed to evaluate the viability and necrosis of HCC accurately for optimally choosing the further proper managing methods. Angiography is an effective method for evaluating HCC lesions treated with TACE. It could reflect sufficiently the blood supply of viable tumors and demonstrate the blood supply of lateral circulation of HCC. But angiography is an invasive technique and therefore is not suitable for routine follow-up in such patients[40,41]. CT could be considered as a routine modality to judge the efficacy of TACE. It could demonstrate accurately the size, shape, location of the lesions, intrahepatic metastasic nodules and the distribution of lipiodol in the tumors and provide valuable imaging information to determinate the interval of TACE[42-49]. Generally, the homogeneous and complete deposition of lipiodol within the lesions would indicate the high degree necrosis of the tumors, but it is difficult to judge the viability and necrosis of the tumors correctly, due to the inhomogeneous deposition, because lipiodol negative area doesn't actually represent the viability of the tumors. The necrosis within the lesions before TACE was also lipiodol negative area. On the other hand, the viable tumors could be enhanced on the CT contrast scanning, but the enhancement area within the lesions could also be affected by artifacts of the high concentrations of lipiodol, making it difficult to evaluate the therapeutic efficiency objectively.
Several authors considered that MR was valuable in the evaluation of therapeutic efficiency of TACE, especially on SE T2WI, most of viable tumors were hyperintense and the coagulative necrosis within the tumors considered as a positive response to TACE were hypointense[50-58]. But this results showed that the signal intensity of the tumors after TACE were variable on the SE T1WI and T2WI, but all of viable tumors, hemorrhage, liquefied necrosis and inflammatory infiltration could also result in hyperintensity on the T2WI. Therefore it was difficult to assess the viable tumors of HCC after TACE by conventional SE imaging. However, it was reliable to judge coagulative necrosis on T2WI, especially the changes during the process of intratumor hemorrhage after TACE presenting as hyperintensity and then turned in to coagulative necrosis presenting hypointensity. This study also demonstrated that it was significant to compare the signal intensity of HCC on T2WI before and after TACE to evaluate the degree of coagulative necrosis. The original hyperintensity of HCC turned to hypointensity indicated the presence of coagulative necrosis after TACE.
FMPSPGR dynamic contrast scanning plays a very important role in the detection and characterization of HCC. It is possible to obtain the high quality images of whole liver during a single breath-hold with rapid aquisition. It could demonstrate accurately the blood supply of tumors and reveal the contrast enhancement patterns of HCC. HCC is hypervascular and enhanced rapidly and obviously at the dynamic early phase scanning and declined at the late phase[33-39]. This results showed that FMPSPGR dynamic contrast scanning also had a great value in the evaluation of therapeutic efficacy of TACE. The residual viable tumors were showed as rapid enhanced portions within the lesions, homogeneous or inhomogeneous, when necrotic portions had no enhancement at the contrast early phase scanning. At the late phase scanning, the enhancement of the most lesions became hypointensity, and just a few lesions showed persistent enhancement. Pathologically, both viable tumors and inflammatory infiltration could present such changes, so the contrast early phase scanning was more reliable in the evaluation of viable tumors, combined by with conventional SE sequence, and more accurate to assess the viability and the necrosis of tumors and useful in the followed up of HCC patients after TACE.
This study has some limitation in which all MR images based on histological specimens, but false positivity or false negativity may be present because of the factors in sampling.
Edited by Zhang JZ
| 1. | Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002;8:193-199. [PubMed] |
| 2. | Wu MC, Shen F. Progress in research of liver surgery in China. World J Gastroenterol. 2000;6:773-776. [PubMed] |
| 3. | Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, Zhou XD, Ye SL. Multimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal vein. World J Gastroenterol. 2001;7:28-32. [PubMed] |
| 4. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] |
| 5. | Parks RW, Garden OJ. Liver resection for cancer. World J Gastroenterol. 2001;7:766-771. [PubMed] |
| 6. | Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, Guo YP, Lin XJ, Li GH. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002;8:74-78. [PubMed] |
| 7. | Palma LD. Diagnostic imaging and interventional therapy of hepatocellular carcinoma. Br J Radiol. 1998;71:808-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Livraghi T. Treatment of hepatocellular carcinoma by interventional methods. Eur Radiol. 2001;11:2207-2219. [PubMed] |
| 9. | Gattoni F, Dova S, Uslenghi CM. Three-year follow-up of 62 cirrhotic patients with hepatocellular carcinoma treated with chemoembolization. Minerva Chir. 2000;55:31-37. [PubMed] |
| 10. | Luo YQ, Wang Y, Chen H, Wu MC. Effect of post-operative survival rate with pre-operative transcantheter arterial chemoembolization in the patients with resectable hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2001;9:468-469. |
| 11. | Zangos S, Mack MG, Straub R, Engelmann K, Eichler K, Balzer J, Vogl TJ. [Transarterial chemoembolization (TACE) of liver metastases. A palliative therapeutic approach]. Radiologe. 2001;41:84-90. [PubMed] |
| 12. | Tu SP, Wu DM, Yuan YZ, Wu YL, Jiang SH, Wu XX. Treatment of hepatocellular carcinoma by transcantheter arterial chemoembolization with hydroxycamptothecin. Shijie Huaren Xiaohua Zazhi. 1999;7:158-160. |
| 13. | Cao W, Wang ZM, Liang ZH, Zhang HX, Wang YQ, Guan Y, Li WX, Pan BR. Effects of angiogenesis inhibitor TNP-470 with lipiodol in arterial embolization of liver cancer in rabbits. Shijie Huaren Xiaohua Zazhi. 2000;8:629-632. |
| 14. | Jia XC, Tian JM, Wang ZT, Chen D, Ye H, Liu Q, Yang JJ, Sun F, Lin L, Lu JP. A retrospective review on interventional treatment of 10000 cases of liver cancer. Shijie Huaren Xiaohua Zazhi. 1998;6:2-3. |
| 15. | Wang HL, Zhao XX, Chen RP, Jing RF, Zhang XP. Treatment of hepatocellular carcinoma by hepatic arterial chemoembolization in 58 cases. Xin Xiaohua Bingxue Zazhi. 1996;4:471. |
| 16. | Zheng CS, Feng GS, Zhou RP, Liang B, Liang HP, Zhen J, Yu JM, Liu H. Hepatic arterial infution chemotherapy and embolization in the treatment of primary hepatic carcinoma. China Natl J New Gastroenterol. 1997;3:104-107. |
| 17. | Lencioni R, Paolicchi A, Moretti M, Pinto F, Armillotta N, Di Giulio M, Cicorelli A, Donati F, Cioni D, Bartolozzi C. Combined transcatheter arterial chemoembolization and percutaneous ethanol injection for the treatment of large hepatocellular carcinoma: local therapeutic effect and long-term survival rate. Eur Radiol. 1998;8:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Fan J, Ten GJ, He SC, Guo JH, Yang DP, Wang GY. Arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 1998;4:33-37. [PubMed] |
| 20. | Li L, Wu PH, Li JQ, Zhang WZ, Lin HG, Zhang YQ. Segmental transcatheter arterial embolization for primary hepatocellular carcinoma. World J Gastroenterol. 1998;4:511-512. [PubMed] |
| 21. | Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Dao T, Van Steenbergen W, Buffet C, Rougier P, Adler M. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129-134. [PubMed] |
| 22. | Allgaier HP, Deibert P, Olschewski M, Spamer C, Blum U, Gerok W, Blum HE. Survival benefit of patients with inoperable hepatocellular carcinoma treated by a combination of transarterial chemoembolization and percutaneous ethanol injection--a single-center analysis including 132 patients. Int J Cancer. 1998;79:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Wang JH, Lin G, Yan ZP, Wang XL, Cheng JM, Li MQ. Stage II surgical resection of hepatocellular carcinoma after TAE: a report of 38 cases. World J Gastroenterol. 1998;4:133-136. [PubMed] |
| 24. | Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, Vilana R, Rodés J. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 391] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Huang J, He X, Lin X, Zhang C, Li J. Effect of preoperative transcatheter arterial chemoembolization on tumor cell activity in hepatocellular carcinoma. Chin Med J (Engl). 2000;113:446-448. [PubMed] |
| 26. | Kuyvenhoven JPh CB, van Hoek B. Practical management of hepatocellular carcinoma. Scand J Gastroenterol Suppl. 2001;234:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Koda M, Murawaki Y, Mitsuda A, Oyama K, Okamoto K, Idobe Y, Suou T, Kawasaki H. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer. 2001;92:1516-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 28. | Poyanli A, Rozaneş I, Acunaş B, Sencer S. Palliative treatment of hepatocellular carcinoma by chemoembolization. Acta Radiol. 2001;42:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Loewe C, Cejna M, Schoder M, Thurnher MM, Lammer J, Thurnher SA. Arterial embolization of unresectable hepatocellular carcinoma with use of cyanoacrylate and lipiodol. J Vasc Interv Radiol. 2002;13:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Alsowmely AM, Hodgson HJ. Non-surgical treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2002;16:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Dohmen K, Shirahama M, Shigematsu H, Miyamoto Y, Torii Y, Irie K, Ishibashi H. Transcatheter arterial chemoembolization therapy combined with percutaneous ethanol injection for unresectable large hepatocellular carcinoma: an evaluation of the local therapeutic effect and survival rate. Hepatogastroenterology. 2001;48:1409-1415. [PubMed] |
| 32. | Zhou KR, Yan FH. Imaging diagnosis of micro liver cancer in China. Shijie Huaren Xiaohua Zazhi. 2001;9:733-736. |
| 33. | Oi H, Murakami T, Kim T, Matsushita M, Kishimoto H, Nakamura H. Dynamic MR imaging and early-phase helical CT for detecting small intrahepatic metastases of hepatocellular carcinoma. AJR Am J Roentgenol. 1996;166:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Siegelman ES, Outwater EK. MR imaging techniques of the liver. Radiol Clin North Am. 1998;36:263-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Fernandez MP, Redvanly RD. Primary hepatic malignant neoplasms. Radiol Clin North Am. 1998;36:333-348. [PubMed] |
| 36. | Yan F, Zhou K, Shen J. [Comparison of enhancement patterns of multi-phase scan of dynamic MRI and dynamic CT in small hepatocellular carcinoma]. Zhonghua Zhongliu Zazhi. 2001;23:413-416. [PubMed] |
| 37. | Yan F, Zhou K, Wu D, Yang J, Gong J, Shen J. [Evaluation of dynamic enhanced fast multiplanar spoiling gradient recalled (FMPSPGR) in the diagnosis of small hepatocellular carcinoma]. Zhonghua Ganzangbing Zazhi. 2001;9:139-141. [PubMed] |
| 38. | Yan FH, Yang J, Zeng MS, Zhou KR. Comparative study of spiral CT, dynamic MRI and US for diagnosis of small hepatocellular carcinoma. Zhongguo Yixue Jisuanji Chengxiang Zazhi. 1997;3:20-24. |
| 39. | Onaya H, Itai Y. MR imaging of hepatocellular carcinoma. Magn Reson Imaging Clin N Am. 2000;8:757-768. [PubMed] |
| 40. | Tsui EY, Chan JH, Cheung YK, Cheung CC, Tsui WC, Szeto ML, Lau KW, Yuen MK, Luk SH. Evaluation of therapeutic effectiveness of transarterial chemoembolization for hepatocellular carcinoma: correlation of dynamic susceptibility contrast-enhanced echoplanar imaging and hepatic angiography. Clin Imaging. 2000;24:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Gattoni F, Dova S, Tonolini M, Uslenghi CM. [Study of the liver and the portal venous system with digital rotational angiography]. Radiol Med. 2001;101:118-124. [PubMed] |
| 42. | Choi BI, Kim HC, Han JK, Park JH, Kim YI, Kim ST, Lee HS, Kim CY, Han MC. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992;182:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, Sato M, Yamanaka N, Shimamura Y, Ohto M. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 44. | Vogl TJ, Trapp M, Schroeder H, Mack M, Schuster A, Schmitt J, Neuhaus P, Felix R. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Katyal S, Oliver JH, Peterson MS, Chang PJ, Baron RL, Carr BI. Prognostic significance of arterial phase CT for prediction of response to transcatheter arterial chemoembolization in unresectable hepatocellular carcinoma: a retrospective analysis. AJR Am J Roentgenol. 2000;175:1665-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Lencioni R, Pinto F, Armillotta N, Di Giulio M, Gaeta P, Di Candio G, Marchi S, Bartolozzi C. Intrahepatic metastatic nodules of hepatocellular carcinoma detected at lipiodol-CT: imaging-pathologic correlation. Abdom Imaging. 1997;22:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Marcato N, Abergel A, Alexandre M, Boire JY, Darcha C, Duchène B, Chipponi J, Boyer L, Viallet JF, Bommelaer G. [Hepatocellular carcinoma in cirrhosis: semeiology and performance of magnetic resonance imaging and lipiodol computed tomography]. Gastroenterol Clin Biol. 1999;23:114-121. [PubMed] |
| 48. | Catalano O, Esposito M, Sandomenico F, Nunziata A, Siani A. [Multiphasic helical computerized tomography of hepatocarcinoma. Assessment after chemoembolization]. Radiol Med. 2000;99:456-460. [PubMed] |
| 49. | Sze DY, Razavi MK, So SK, Jeffrey RB. Impact of multidetector CT hepatic arteriography on the planning of chemoembolization treatment of hepatocellular carcinoma. AJR Am J Roentgenol. 2001;177:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Castrucci M, Sironi S, De Cobelli F, Salvioni M, Del Maschio A. Plain and gadolinium-DTPA-enhanced MR imaging of hepatocellular carcinoma treated with transarterial chemoembolization. Abdom Imaging. 1996;21:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Ito K, Honjo K, Fujita T, Matsui M, Awaya H, Matsumoto T, Matsunaga N, Nakanishi T. Therapeutic efficacy of transcatheter arterial chemoembolization for hepatocellular carcinoma: MRI and pathology. J Comput Assist Tomogr. 1995;19:198-203. [PubMed] |
| 52. | Yamashita Y, Yoshimatsu S, Sumi M, Harada M, Takahashi M. Dynamic MR imaging of hepatoma treated by transcatheter arterial embolization therapy. Assessment of treatment effect. Acta Radiol. 1993;34:303-308. [PubMed] |
| 53. | De Santis M, Torricelli P, Cristani A, Cioni G, Montanari N, Sardini C, Ventura E, Romagnoli R. MRI of hepatocellular carcinoma before and after transcatheter chemoembolization. J Comput Assist Tomogr. 1993;17:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Bartolozzi C, Lencioni R, Caramella D, Falaschi F, Cioni R, DiCoscio G. Hepatocellular carcinoma: CT and MR features after transcatheter arterial embolization and percutaneous ethanol injection. Radiology. 1994;191:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | De Santis M, Alborino S, Tartoni PL, Torricelli P, Casolo A, Romagnoli R. Effects of lipiodol retention on MRI signal intensity from hepatocellular carcinoma and surrounding liver treated by chemoembolization. Eur Radiol. 1997;7:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Semelka RC, Worawattanakul S, Mauro MA, Bernard SA, Cance WG. Malignant hepatic tumors: changes on MRI after hepatic arterial chemoembolization--preliminary findings. J Magn Reson Imaging. 1998;8:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Kubota K, Hisa N, Nishikawa T, Fujiwara Y, Murata Y, Itoh S, Yoshida D, Yoshida S. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom Imaging. 2001;26:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Chan JH, Tsui EY, Luk SH, Yuen MK, Cheung YK, Wong KP. Detection of hepatic tumor perfusion following transcatheter arterial chemoembolization with dynamic susceptibility contrast-enhanced echoplanar imaging. Clin Imaging. 1999;23:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |