Published online Apr 15, 2002. doi: 10.3748/wjg.v8.i2.353
Revised: November 7, 2001
Accepted: November 14, 2001
Published online: April 15, 2002
AIM: To isolate a novel isoform of human HPO (HPO-205) from human fetal liver Marathon-ready cDNA and characterize its primary biological function.
METHODS: 5'-RACE (rapid amplification of cDNA 5’ends) was used to isolate a novel isoform of hHPO in this paper. The constructed pcDNAHPO-205, pcDNAHPO and pcDNA eukaryotic expression vectors were respectively transfected by lipofectamine method and the stimulation of DNA synthesis was observed by 3H-TdR incorporation assay. Proteins extracted from different cells were analyzed by Western blot.
RESULTS: A novel isoform of hHPO (HPO-205) encoding a 205 amino acid ORF corresponding to a translated production of 23 kDa was isolated and distinguished from the previous HPO that lacked the N-terminal 80 amino acids. The dose-dependent stimulation of DNA synthesis of HepG2 hepatoma cells by HPO-205 demonstrated its similar biological activity with HPO in vitro. The level of MAPK (Mitogen-activated protein kinase) phosphorylation by Western blot analysis revealed that HPO-205 might have the stronger activity of stimulating hepatic cell proliferation than that of HPO.
CONCLUSION: A novel isoform of hHPO (HPO-205) was isolated from hepatic-derived cells. The comparison of HPO-205 and HPO will lead to a new insight into the structure and function of hHPO, and provide the new way of thinking to deeply elucidate the biological roles of HPO/ALR.
- Citation: Lu J, Xu WX, Zhan YQ, Cui XL, Cai WM, He FC, Yang XM. Identification and characterization of a novel isoform of hepatopoietin. World J Gastroenterol 2002; 8(2): 353-356
- URL: https://www.wjgnet.com/1007-9327/full/v8/i2/353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i2.353
Hepatic stimulatory activity was identified from human fetal liver lysate, which was named as hepatopoietin (HPO)[1]. Later we proved that HPO was encoded by mRNA of human fetal liver[2]. Recombinant human hepatopoietin (rhHPO) showed its activity of specifically stimulating DNA synthesis of hepatic cells and promoting healing after liver injury in vitro and in vivo[3-6]. Recently, HPO/ALR/EVR1 homologous cDNAs (including EST sequences) have been isolated in many laboratories[7,8]. Sequence analysis of these homologous cDNAs revealed that the same 3' sequence was presented in all reported sequences, however, the 5' sequence varied in these cDNAs[9]. Furthermore, The analysis of genomic sequences of HPO/ALR also suggested there might exist different transcripts in nature. In this paper, we reported the identification and characterization of a novel isoform of human HPO in hepatic-derived cells. This cDNA with a 205 amino acid ORF was named HPO-205 to distinguish it from the previous HPO that lacked the N-terminal 80 amino acids.
HPO sequences and their proteins were analyzed by using DNA tools, Biosoft software and GenBank blast program. The primer for 5'RACE was: 5'-GGTCTTCAGGTTCAGACACATGTTGGC-3'. The human fetal liver Marathon-ready cDNA Clontech) served as template to amplify 5’end of HPO by using a kit (Clontech). The PCR products were ligated to pGEM-T vector and sequenced using T7 and SP6 primers by PE (ABI PRISM) DNA Sequencer. Cos7 (African green monkey kidney cell line), HepG2 and HLE (human hepatoma cell line) were purchased from the American Type Culture Collection. BEL-7402 (epithelial hepatoma cell lines, derived from male, 75 years) and SMMC-7721 (epithelial hepatoma cell lines, derived from male, 50 years) were purchased from the Cell Institute of Chinese Academy of Science. All cell lines were cultured in Dulbecco's Modified Eagle Media (DMEM) (GiBco BRL, Life Technologies.) supplemented with antibiotics and 100 mL•L⁻¹ heat-inactivated fetal bovine serum in a humidified atmosphere of 50 mL•L⁻¹ CO2.Total RNA was isolation from the cells using TRIzolTM reagent (GiBco).
Forward and reverse primers for human HPO-250 and G3PDH genes were designed and applied to amplify transcripts. Briefly, Total RNAs were extracted with Trizol (GIbco) reagent. Reverse-transcribed and amplified for 30 cycles, using RT and PCR kit (TaKaRa LA Taq with GC buffer, TaKaRa Biotech, Dalian, China), according to the manufacturer's instructions. The expression of G3PDH mRNA was detected as an internal control. Primer pairs for RT-PCR were: HPO-250 sense: 5'-ATGGCGGCGCCCGGCGAGCGGGGCCGCTT-3'; antisense: 5'-CTAGTCACAGGAGCCATCCTTCCA-3'; G3PDH sense: 5'-ACCACAGTCCATGCCATCAC-3'; antisense: 5'-TCCACCACCCTGTTGCTGTA-3'. The expected size of the amplified DNA fragments for HPO-250 and G3PDH were 618 and 1000 bp respectively. The PCR products were separated on 10 g•L⁻¹ agarose gel with ethidium bromide staining and photographed under UV. The photographs of the gels were analysed using Bio-Print (M&S Instruments Trading Inc, Tokyo). The values of these DNA fragments were calculated as relative intensity against G3PDH mRNA.
Both EcoR I and BamH I sites were introduced by PCR in vitro mutagenesis, and the full length cDNA encoding human HPO and HPO-250 were inserted into pcDNA 3.1(+) vector downstream of CMV promoter. The recombinant pCDNA 3.1 plasmids were isolated and used for transfection. Cos-7 or Bel-7402 cells were subsequently harvested and reseeded at a density of 1 × 106 cells/100-mm plate. The cells were transfected the next day with different plasmids (2 μg) using lipofectamine reagent (Gibco). For transient expression, the conditional medium of Cos-7 cells transfected with pcDNAHPO-250 or pcDNA plasmid was harvested 48 h after transfection. For stable expression, the Bel-7402 cells after being transfected with pcDNAHPO-250 or pcDNAHPO or pcDNA plasmid by 72 h were selected in DMEM medium containing 400 mg•L⁻¹ G418 for 14 d. G418-resistant clones were isolated and grown in DMEM medium containing G418 to maintain the phenotype.
Protein was extracted from different cells, and the protein concentration was determined by Coomassie Brilliant Blue G-250 staining. Samples containing equivalent amounts (50 μg) were separated on a 100 g•L⁻¹ SDS polycocrylamide gel under reducing conditions and transferred to a Hybond-N nitrocellulose membrane (Amersham, Arlington Heights, Illinois). The membrane was incubated respectively with rabbit polyclonal anti-human HPO antibody or rabbit polyclonal anti-pERK or ERK antibody (Santa Cruz) and developed with an ECL western blotting detection system (Santa Cruz) using horseradish peroxidase-conjugated second antibody (Santa Cruz).
HepG2 hepatoma cells were counted and adjusted to 1 × 108 cells•L⁻¹, then 100 μL of cell suspension was inoculated into 96-well plates and incubated at 37 °C, 50 mL•L⁻¹ CO2 for 12 h. Then the fresh medium containing prepared samples was added. After 48 h of culture in presence of various medium supplements, 37 kBq/well 3H-TdR was added and incubated for 3 h. The cells were collected to filters, and radioactivity was determined in an LKB liquid scintillation counter, and results were expressed as median counts per minute from triplicate cultures.
Searching against GenBank with human HPO sequence, this sequence showed homology with 11 human cDNAs encoding HPO-like proteins, Protein sequence alignment showed that all of these encoding proteins were highly conserved in the 125 amino acid of C terminal but varied in the N terminal. Further analysis of their nucleotide sequences demonstrated no stop codon was found before the same open reading frame (ORF). This result indicated that deposited 125 amino acid HPO might be incomplete.
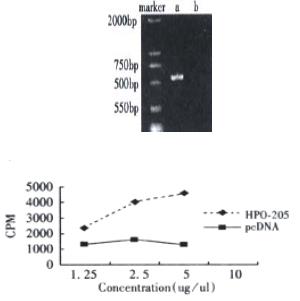
To investigate whether a novel isoform of HPO exists in nature, a Marathon-ready cDNA from human fetal liver served as the template to amplify 5’ends of hHPO. The PCR products containing different sizes of 375, 500, 750 and 1200 bp bands were cloned into pMD18-Tvectors and transformed Ecoli. DH5α. Then the clones were screened by hybridization with 32P-HPO cDNA as a probe, and more than 50 positive clones were obtained. After we sequenced 10 positive clones, we obtained the novel isoform HPO cDNA that encoded a 205 amino acid ORF. This cDNA with a 205 amino acid ORF was named HPO-205 (GenBank CI 11559825) to distinguish it from the previous HPO that lacked the N-terminal 80 amino acids (Figure 1).
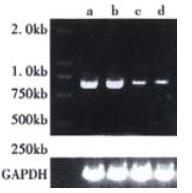
The expression of HPO-205 was determined by semi-quantitive RT-PCR and Western blotting. As shown in Figure 2 and Figure 3, the cell lines showed interesting and revealing differences in the levels of HPO-205. The levels of hHPO-205 mRNA in HepG2 and HLE cell lines were significantly higher (4-5 folds) than in BEL-7402 and 7721 cell lines (Figure 2). Similarly, we also observed HPO-205 protein in HepG2 and HLE cell lines were significantly higher (4-5 folds) than in BEL-7402 and 7721 cell lines (Figure 3) by Western blot analysis. The Mr of HPO-205 in these four hepatoma cell lines is 23000 identical to the predicted molecular weight (Figure 3).
We demonstrated that biological activity of HPO-205 could be expressed from its cDNA in transient expression experiment in Cos7 cells. As shown in Figure 4A and Figure 4B, the conditional medium from transfected cells with the pcDNAHPO-205 revealed the dose-dependent stimulation of DNA synthesis of HepG2 hepatoma cells. However, as a negative control, the conditional medium from mock-transfected did not show any activity.
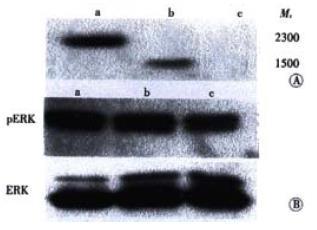
MAPK could be activated by a number of growth factors and cytokines. To assess whether HPO-205 activated the MAPK pathway, the pcDNAHPO-205, pcDNAHPO and pcDNA plasmids were transfected into Bel-7402 cells with low level expression of HPO. Expression of HPO-205 and HPO protein were analyzed by western blotting using antibody against HPO protein. As shown in Figure 5A, a protein band with an apparent Mr of 23000 only was detected in the HPO-205 transfected Bel-7402 cells, and a protein band with an apparent Mr of 15000 only was detected in the HPO transfected Bel-7402 cells. As shown in Figure 5B, the level of MAPK phosphorylation in BEL-7402 cells could be elevated by HPO and HPO-205 (P < 0.01), and the level of MAPK phosphorylation activated by HPO-205 was higher than that by HPO (P < 0.01).
We isolated and characterized a novel isoform of HPO (HPO-205) cDNA that encoded a 205 amino acid ORF. This cDNA with a 205 amino acid ORF was named HPO-205 (GenBank CI 11559825) to distinguish it from the previous HPO that lacked the N-terminal 80 amino acids. The molecular weight of HPO-205 was 23 kDa identified in four human hepatoma cell lines by Western blot, and the HPO-205 mRNA expressions were detectable in human hepatoma cell lines including HepG2, HLE, 7402 and 7721 by RT-PCR method. Our sequence analysis of HPO-205 shows presence of very rich GC bases in 5’end of HPO mRNA (more than 75%). Such high amount of GC bases will affect the efficiency of mRNA transcription. Therefore, it will lead to obtaining different kinds of incompleted HPO mRNA under different conditions in many laboratories[10].
As we know, human HPO is a specific growth factor and plays an important role in liver regeneration in vivo[11-14]. Many experiments demonstrated that HPO could stimulate the proliferation of hepatic cells in vitro and in vivo[1,2,15]. Therefore, identification and characterization of the biological function of HPO-205 and the relationship between HPO-205 and HPO are very important. Some data have shown they are different in intracellular location, tissue distribution and mRNA expression under different pathological conditions, which have suggested their different biological functions[16]. In this paper, we find, as human HPO, HPO-205 also stimulated the DNA synthesis of HepG2 cells. However, whether HPO-205 can specifically stimulate DNA synthesis of hepatic cells and promote healing after liver injury in vitro and in vivo, just like HPO, remain to be under investigation.
Our previous study demonstrated that HPO stimulated the hepatic cell proliferation through its specific receptor[4]. Recently, another report indicates that HPO/ALR assigns a FAD-linked sulfhydryl oxidase activity, which plays a role in modifying some molecules in vivo[17], and regulates the expression of some mitochondrial genes[18-21]. These data suggest that there are many different mechanisms of HPO/ALR involved in stimulating cell proliferation and improving hepatic repair. Recently, Li et al[22] have reported that HPO promotes the proliferation of hepatic cells through activating MAPK signal pathway. Here we have also found that both of HPO-205 and HPO could increase the activation of MAPK phosphorylation compared with the control. Moreover, the result also showed that the level of MAPK phosphorylation in HPO-205 transfected cells was significantly higher than that in HPO transfected cells by Western blot. These data indicate that HPO-205 might have the stronger activity of stimulating hepatic cell proliferation than that of HPO.
Edited by Hu DK
| 1. | Yang X, Xie L, Qiu Z, Wu Z, He F. Human augmenter of liver regeneration: Molecular cloning, biological activity and roles in liver regeneration. Sci China C Life Sci. 1997;40:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Yang XM, Wang AM, Zhou P, Xie L, Wang QM, Wu ZZ, He FC. Human hepatopoietin--A hepatotrophic factor or liver regeneration, and its potential antihepatitis effect in vivo. Chin Sci Bull. 1998;43:1026-1031. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 3. | Yang XM, Hu ZY, Xie L, Wu ZZ, Wu CT, He FC. [In vitro stimulation of HTC hepatoma cell growth by recombinant human augmenter of liver regeneration (ALR)]. Shengli Xuebao. 1997;49:557-561. [PubMed] |
| 4. | Wang G, Yang X, Zhang Y, Wang Q, Chen H, Wei H, Xing G, Xie L, Hu Z, Zhang C. Identification and characterization of receptor for mammalian hepatopoietin that is homologous to yeast ERV1. J Biol Chem. 1999;274:11469-11472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Yang X, Wang A, Zhou P, Wang Q, Wei H, Wu Z, He F. Protective effect of recombinant human augmenter of liver regeneration on CCl4-induced hepatitis in mice. Chin Med J (. Engl). 1998;111:625-629. [PubMed] |
| 6. | Tanigawa K, Sakaida I, Masuhara M, Hagiya M, Okita K. Augmenter of liver regeneration (ALR) may promote liver regeneration by reducing natural killer (NK) cell activity in human liver diseases. J Gastroenterol. 2000;35:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Cheng J, Zhong Y. [Cloning and sequence analysis of human genomic DNA of augmenter of liver regeneration hepatitis]. Zhonghua Ganzangbing Zazhi. 2000;8:12-14. [PubMed] |
| 8. | Dong J, Cheng J, Liu Y, Wang Q, Wang G, Shi S, Si C. [Cloning and sequence analysis of a pseudogene of liver regeneration augmenter in rats]. Zhonghua Ganzangbing Zazhi. 2001;9:105-107. [PubMed] |
| 9. | Hofhaus G, Stein G, Polimeno L, Francavilla A, Lisowsky T. Highly divergent amino termini of the homologous human ALR and yeast scERV1 gene products define species specific differences in cellular localization. Eur J Cell Biol. 1999;78:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Yi XR, Kong XP, Tong MH, Yang LP, Li RB, Zhang YJ. Cloning and sequencing of rat and human augmenter of liver regeneration gene. Shijie Huaren Xiaohua Zazhi. 1998;6:392-393. |
| 11. | Gandhi CR, Kuddus R, Subbotin VM, Prelich J, Murase N, Rao AS, Nalesnik MA, Watkins SC, DeLeo A, Trucco M. A fresh look at augmenter of liver regeneration in rats. Hepatology. 1999;29:1435-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Francavilla A, Vujanovic NL, Polimeno L, Azzarone A, Iacobellis A, Deleo A, Hagiya M, Whiteside TL, Starzl TE. The in vivo effect of hepatotrophic factors augmenter of liver regeneration, hepatocyte growth factor, and insulin-like growth factor-II on liver natural killer cell functions. Hepatology. 1997;25:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Shen M, Qiu DH, Chen Y, Xiong WJ. Effects of recombinant augmenter of liver regeneration protein, danshen and oxymatrine on rat fibroblasts. Shijie Huaren Xiaohua Zazhi. 2001;9:1129-1133. |
| 14. | Zhou P, Yang XM, Li QF, He H, He FC, Zhang MS. Detection of augmenter of liver regeneration in sera of patients with various liver disease. Shijie Huaren Xiaohua Zazhi. 1998;6:768-770. |
| 15. | Yang XM, Xie L, Xing GC, Wu ZZ, He FC. Partial isolation and identification of hepatic stimulator substance mRNA extracted from human fetal liver. World J Gastroenterol. 1998;4:100-102. [PubMed] |
| 16. | Lu C, Li Y, Zhao Y, Xing G, Tang F, Wang Q, Sun Y, Wei H, Yang X, Wu C. Intracrine hepatopoietin potentiates AP-1 activity through JAB1 independent of MAPK pathway. FASEB J. 2002;16:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Lisowsky T, Lee JE, Polimeno L, Francavilla A, Hofhaus G. Mammalian augmenter of liver regeneration protein is a sulfhydryl oxidase. Dig Liver Dis. 2001;33:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Lee J, Hofhaus G, Lisowsky T. Erv1p from Saccharomyces cerevisiae is a FAD-linked sulfhydryl oxidase. FEBS Lett. 2000;477:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Polimeno L, Capuano F, Marangi LC, Margiotta M, Lisowsky T, Ierardi E, Francavilla R, Francavilla A. The augmenter of liver regeneration induces mitochondrial gene expression in rat liver and enhances oxidative phosphorylation capacity of liver mitochondria. Dig Liver Dis. 2000;32:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Lange H, Lisowsky T, Gerber J, Mühlenhoff U, Kispal G, Lill R. An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep. 2001;2:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Polimeno L, Margiotta M, Marangi L, Lisowsky T, Azzarone A, Ierardi E, Frassanito MA, Francavilla R, Francavilla A. Molecular mechanisms of augmenter of liver regeneration as immunoregulator: its effect on interferon-gamma expression in rat liver. Dig Liver Dis. 2000;32:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Li Y, Li M, Xing G, Hu Z, Wang Q, Dong C, Wei H, Fan G, Chen J, Yang X. Stimulation of the mitogen-activated protein kinase cascade and tyrosine phosphorylation of the epidermal growth factor receptor by hepatopoietin. J Biol Chem. 2000;275:37443-37447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |