Published online Apr 15, 2002. doi: 10.3748/wjg.v8.i2.294
Revised: June 3, 2001
Accepted: July 10, 2001
Published online: April 15, 2002
AIM: To characterize the biochemical and immunological properties of an experimental ISCOMS vaccine prepared from a novel therapeutic polypeptide based on T cell epitopes of HBsAg, and a heptatis B-ISCOMS was prepared and investigated.
METHODS: An immunostimulating complexes (ISCOMS)-based vaccine containing a novel therapeutic hepatits B polypeptide was prepared by dialysis method, and its formation was visualized by electron microscopy and biochemically verified by SDS-polyacrylamide gel electrophoresis. Amount of the peptide within ISCOMS was determined by Bradford assay, and specific CTL response was detected by ELISPOT assay.
RESULTS: Typical cage-like structures of submicroparticle with a diameter of about 40 nm were observed by electron microscopy. Results from Bradford assay showed that the level of peptide incorporation was about 0.33 g•L⁻¹. At the paralleled position close to the sixth band of the molecular weight marker (3480 kDa) a clear band was shown in SDS-PAGE analysis, indicating successful incorporation of polypeptide into ISCOMS. It is suggested that ISCOMS delivery system could efficiently improve the immunogenicity of polypeptide and elicit specific immune responses in vivo by the results of ELISPOT assay, which showed that IFN-γ producing cells (specific CTL responses) were increased (spots of ISCOMS-treated group: 47 ± 5, n = 3; control group: 5 ± 2, n = 3).
CONCLUSION: ISCOMS-based hepatitis B polypeptide vaccine is successfully constructed and it induces a higher CTL response compared with short polypeptides vaccine in vivo.
- Citation: Guan XJ, Guan XJ, Wu YZ, Jia ZC, Shi TD, Tang Y. Construction and characterization of an experimental ISCOMS-based hepatitis B polypeptide vaccine. World J Gastroenterol 2002; 8(2): 294-297
- URL: https://www.wjgnet.com/1007-9327/full/v8/i2/294.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i2.294
Infection of hepatitis B virus (HBV) is very common in China[1-25], and nearly 100 million people have a persistent infection with HBV who are at risk of developing chronic pepatitis leading to liver cirrhosis and hepatocellular carcinoma. Up to now, vaccination is a main way in prevention[26-36]. Based on our knowledge and work on epitopes of HBV natural nucleocapsids, using SGI O2 workstation and InsightIIsoftware modeling the configuration of natural HBV PreS2, HBsAg and HBcAg, we have screened out several novel HBV therapeutic polypeptides containing immunodominant B-, T helper (Th) and cytotoxic T lymphocyte responses (CTL) epitopes of HBV PreS2, HBsAg and HBcAg. It is well known that natural antigens and their dominant epitope peptides can not induce sufficient antigen-specific CTL responses in vivo, although they could pulse antigen-specific CTLs in vitro. Therefore efforts should be made to potently promote or enhance their antigenicity so as to induce efficient immune responses including CTLs in vivo, among which utilization of appropriate adjuvants or delivery systems is a promising and useful strategy.
Elaborate work has demonstrated that ISCOMS, or immunostimulating complexes, first described by Sweden scientist Bror Morein and his colleagues in 1984, is a good vehicle for antigen presentation. Previously antigens used in earlier works in this complexes were isolated from crude molecular components of microbes while is prepared from phosphatidylcholine (or phosphatidylethanamine), cholesterol and glucoside Quil A (also called Spikoside) and antigen molecule now, which are approximately 40 nm submicroscopic cage-like particles (the size is similar to virus particle). ISCOMS formed in the absence of antigen molecules is termed ISCOMS matrix or empty ISCOMS compared to ISCOMS formed in the presence of antigen (s). Elaborate work has demonstrated that ISCOMS is a good vehicle for antigen presentation. Incorporation into ISCOMS not only allows protein antigens to induce strong antibody, major histocompatibility complex (MHC) class II-restricted T cell responses and mucosal immunity, but also allow antigens to enter the endogenous pathway of antigen processing to induce MHC class I-restricted CTL in vivo[37-39]. Immunization with antigens in ISCOMS induces protective immunity against a number of experimental infections, including influenza, toxoplasmosis, measles, feline leukemia, EBV infection, and herpes simplex[40-42]. An ISCOMS-based vaccine against equine influenza was produced and sold by Iscotec AB in Sweden in 1988. Compared with liposomes, ISCOMS structure is much more rigid, three-dimensional with marked symmetry, which is extremely stable under many conditions, including in the intestine, and may be present for long periods of time intra- and extracellularly in lymphoid tissues. On the basis of our previous work on the design and synthesis of HBV epitope-based vaccines, choosing a polypeptide containing both Th and CTL epitopes of HBV as a model antigen, an experimental ISCOMS-based vaccine was constructed and prepared, and its biochemical and immunological properties were then investigated and discussed in this study.
Phosphatidylcholine, cholesterol, and decanoyl-N-methyl-glucamide (Mega-10) were all from Sigma. Quil A was kindly provided by Dr. Erik B Lindblad. SDS molecular mass marker (2500-17000 u)was from Sigma (MWM-100). Hepatitis B polypeptide, glycopeptide and lipopeptide were synthesized by the solid-phase method with an automated peptide synthesizer (431A, Applied Biosystems, Foster City, CA).
Phosphatidylcholine and cholesterol (10 g•L⁻¹ each) were fully dissolved in 200 g•L⁻¹ (in distilled water) Mega10, aliquot and store at low temperature until required.
Dissolve 100 mg Coomassie blue G and 30 mg SDS in 50 mL 950 mL•L⁻¹ ethanol. Add 100 mL of 850 g•L⁻¹ orthophosphoric acid and make up to 1 L with water. Filter before use.
To a solution of hepatitis B polypeptide (1 g•L⁻¹) and Quil A (1 g•L⁻¹), add 100 μL of lipid mixture stock solution and mix thoroughly. After reaction at room temperature for 2 h, the mixture was transferred to a dialysis bag, dialyzed 24 h at room temperature, then at 4 °C for another 48 h. To confirm the presence of typical structure of ISCOMS, a small aliquot (-80 μL) was negatively stained and examined by electron microscopy analysis. Sterilize by filtration through a 0.22 μm filter.
To each 50 μL sample of ISCOMS and to each 50 μL dilution of BSA add 1 mL Bradfords solution. Incubate 5 min at room temperature. Measure the A595 of each sample and BSA standard. Plot a standard curve from the BSA values and determine the protein concentration of the ISCOMS by interpolation from this curve.
Prepare gels as indicated in Table 1. After the gel has set, allow to equilibrate by leaving overnight at 4 °C. Remove the comb and rinse wells with water, then with Cathode Buffer. Load the samples and molecular weight marker. Electrophoresis condition: constant current at 20 mA for 1 h and 30 mA for 4-6 h (the marker dye is within 1 cm of the anodic end of the gel). Immerse in the fixative solution for 30 min. Transfer to staining solution for 1 h and destaining solution for 2 h, renewing the solution every 30 min. The gel now is ready for visualization, analysis or qhotography.
| Components | Stacking gel (mL) | Spacer gel (mL) | Separating gel (mL) |
| Acrylamide solution | 5.00 | 3.05 | 1.00 |
| Gel buffer | 5.00 | 5.00 | 3.10 |
| Glycerol | 1.60 | -- | -- |
| Water | 3.40 | 6.95 | 8.40 |
| Total | 15.00 | 15.00 | 12.50 |
Female Balb/c mice, obtained from the Animal Research Center of Academy of Military Medical Sceinces (Beijing, China) were sc injected to the hind footpad with hepatitis B polypeptide-ISCOMS and hepatitis polypeptide (5 nmol each) alone. After 7 d of the first priming, the animals were boosted and lymph nodes were removed 7 d later to prepare single cell suspension for the ELISPOT assay, which was performed according to the instruction of the murine IFN-γ ELISPOT kit (Diaclone, France).
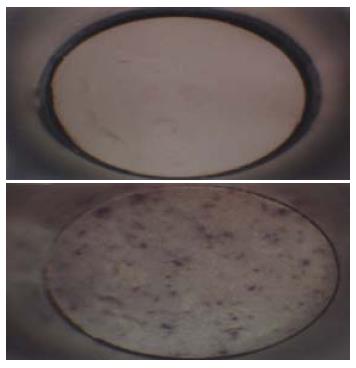
Hepatitis B-ISCOMS unique structure was examined by electron microscopy, which showed uniform honeycomb-like open structure composed of several subunits and confirmed the formation of ISCOMS (Figure 1).
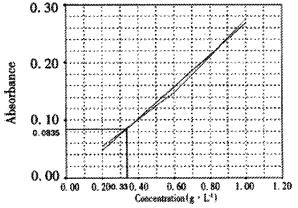
Standard curve was shown as Figure 2 (r = 0.9968). The mean A595 value of ISCOMS was 0.0835. According to the standard curve, the corresponding peptide concentration was about 0.33 g•L⁻¹.
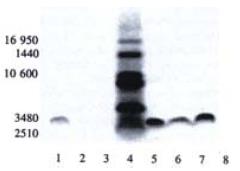
In the SDS-PAGE separation of ISCOMS prepared from different hepatitis B polypeptides we observed that at the paralleled position close to the sixth band of the molecular weight marker there was a clear band indicating the peptide incorporated into ISCOMS (Figure 3). Compared to the polypeptide sample, it was at the similar position but somewhat with a slight tailer, which might be affected by other components in the ISCOMS.
Swelling of lymph nodes were obvious in ISCOMS treated group. In ELISPOT assay, significantly improved IFN-γ linked cytotoxic T lymphocyte (CTL) proliferation response to hepatitis B polypeptide was observed in HBP-ISCOMS group compared to the polypeptide control group (spots of B: 47 ± 5, n = 3; A: 5 ± 2, n = 3, respectively) (Figure 4).
Studies in immunological mechanisms and T cell function have demonstrated that class imajor histocompatibility complex (MHC)-restricted cytotoxic T lymphocytes (CTL) play a critical role in the control of intracellular pathogens and tumor cells growth, but the problems of CTL responses induced in vivo and cell-medicated immunity have not been solved yet. Exogenous soluble antigens are not allowed to enter the endogenous pathway necessary for MHC class I-restricted presentation, therefore are not capable of stimulating MHC class I-restricted CD8+ CTL responses. Obviously, intracellular expression and synthesis of antigens by infection or through pathological/physiological genes could lead to MHC class I restricted-representation pathway, but vaccination of infectants or transfected cells might lead to certain diseases, thus development of adequate novel vaccine adjuvants, especially those themselves are not immunogenic and could present non-replicable soluble antigens to the endogenous pathway and are capable of inducing MHC class I restricted-CTL responses, are badly in demand.
ISCOMS technique is just one of the novel adjuvanted-vaccine systems to meet this demand. Since the first description of ISCOMS appeared more than a decade ago, ISCOMS has been widely used to generate antigen-adjuvant complex in vaccine development, especially in viral antigen studies to promote immune responses. ISCOMS has a unique ability to provoke a full range of immune response to protein antigens, which is efficient after both parenteral and oral immunization. It has a unique ability to allow the antigen molecules to enter the endogenous pathway for antigen processing, which in turn to provoke MHC class I-restricted CTL. It is safe and stable, prepared in mild conditions. Furthermore, as ISCOMS is a non-viable adjuvant vesicle and is not immunogenic and antigenic itself, it could enhance antigen specific immune responses, but not unwanted immunity. Different antigen molecules are able to produce vaccine-adjuvant complex with ISCOMS matrix after proper modification, so are useful and promising in various vaccine design.
Preparations of experimental ISCOMS-based vaccines. have been done with large number of whole microbe or isolated from microbe, especially viruses such as HIV[43-46], influenza virus[38,47-49], EBV[41], HSV[42,50] and Measles[40,51]. The work with the major S gene products (HBsAg) of the hepatitis B virus genome has been reported, but not with epitope-based hepatitis B polypeptide. In our study, on the basis of the molecular design and synthesis of therapeutic heptatitis B peptides previously, different hepatitis B polypeptides were investigated for their abilities to incorporating into ISCOMS.
Formation of typical structure of ISCOMS was verified by electron microscopy. Adequate proportion of the components, extensive dialysis and purification by density gradient ultracentrifugation when necessary, are important factors involved in the preparation of ISCOMS. The presence of typical cage-like microparticles visualized by electron microscopy is a simple and direct method to examine the formation of ISCOMS, but ISCOMS matrix also shows similar structure as ISCOMS containing antigen molecules, so other methods are required to verify the presence of antigen component. Results from Bradford assay suggested that ISCOMS was successfully formed with polypeptide (30) and other components. In our study, polypeptide incorporated into ISCOMS was about 0.33 g•L⁻¹ (incorporation rate 33%). In addition, we observed that the short polypeptide which was easily degraded in a couple of days even at 4 °C, was stable while stored at 4 °C for months without notable degradation, indicating markedly improved stability of antigen peptide after the formation of hepatitis B short polypeptide-ISCOMS.
SDS-PAGE separation of short polypeptide showed an apparent band close to 3480 Da which confirmed the successful incorporation of hepatitis B polypeptide into ISCOMS (peptide failed to incorporate into ISCOMS was removed during dialysis). The peptide control showed a straight and regular band, while those for peptide-ISCOMS were broad which indicated effects of other components on SDS-PAGE separation. Specific CTL responses were markedly enhanced by ISCOMS-adjuvanted hepatitis B vaccine compared to antigen peptide control in mice visualized by ELISPOT assay. More work will be done to investigate the immunological properties and mechanisms underlying of this experimental ISCOMS-based vaccine from hepatitis B polypeptide in vivo.
We are grateful to Dr. Soren Kamstrup and Dr. Erik B Lindblad from Danmark, Dr. Anna Lunden from Sweden and Dr. John Simms from United Kingdom for their kind help in this study.
Edited by Wu XN
| 1. | Wang PZ, Zhang ZW, Zhou YX, Bai XF. Quantitative PCR detection of HBV-DNA in patients with chronic hepatitis B and its significance. Shijie Huaren Xiaohua Zazhi. 2000;8:755-758. |
| 2. | Shi H, Wang FS. Host factors in chronicity of hepatitis B virus infection and their significances clinic alh. Shijie Huaren Xiaohua Zazhi. 2001;9:66-69. |
| 3. | Fang DX, Li FQ, Tan WG, Chen HB, Jin HY, Li SQ, Lin HJ, Zhou ZX. Transient expression and antigenic characterization of HBsAg of HBV nt551 A to G mutant. World J Gastroenterol. 1999;5:73-74. [PubMed] |
| 4. | Guo SP, Ma ZS, Wang WL. Construction of eukaryotic expression vector of HBV x gene. World J Gastroenterol. 1999;5:351-352. [PubMed] |
| 5. | Tang RX, Gao FG, Zeng LY, Wang YW, Wang YL. Detection of HBV DNA and its existence status in liver tissues and peripheral blood lymphocytes from chronic hepatitis B patients. World J Gastroenterol. 1999;5:359-361. [PubMed] |
| 6. | Chen K, Han BG, Ma XK, Zhang HQ, Meng L, Wang GH, Xia F, Song XG, Ling SG. Establishment and preliminery use of hepatitis B virus preS1/2 antigen assay. World J Gastroenterol. 1999;5:550-552. [PubMed] |
| 7. | Qin LL, Su JJ, Li Y, Yang C, Ban KC, Yian RQ. Expression of IGF- II, p53, p21 and HBxAg in precancerous events of hepatocarcinogenesis induced by AFB1 and/or HBV in tree shrews. World J Gastroenterol. 2000;6:138-139. [PubMed] |
| 8. | He XS, Huang JF, Chen GH, Fu Q, Zhu XF, Lu MQ, Wang GD, Guan XD. Orthotopic liver transplantation for fulminant hepatitis B. World J Gastroenterol. 2000;6:398-399. [PubMed] |
| 9. | Hu YP, Yao YC, Li JX, Wang XM, Li H, Wang ZH, Lei ZH. The cloning of 3'-truncated preS/S gene from HBV genomic DNA and its expression in transgenic mice. World J Gastroenterol. 2000;6:734-737. [PubMed] |
| 10. | Wang XZ, Chen XC, Yang YH, Chen ZX, Huang YH, Tao QM. Relationship between HBxAg and Fas/FasL in patients with hepatocellular carcinoma. World J Gastroenterol. 2000;6:17. |
| 11. | Wang SP, Xu DZ, Yan YP, Shi MY, Li RL, Zhang JX, Bai GZ, Ma JX. Hepatitis B virus infection status in the PBMC of newborns of HBsAg positive mothers. World J Gastroenterol. 2000;6:58. |
| 12. | Ma CH, Sun WS, Zhang LN, Ding PF. Inhibitory effect of antisense oligodeoxynucleotides complementary to HBV on HepG2.2.15 cell line. World J Gastroenterol. 2000;6:72. |
| 13. | Gao XW, Jia SY, Liu XM. BCG vaccine combined with dipyridamole in the treatment of HBV infection. World J Gastroenterol. 2000;6:76. |
| 14. | You J, Zhuang L, Tang BZ, Yang H, Yang WB, Li W, Zhang HL, Zhang YM, Zhang L, Yan SM. Interferon alpha with Thymopeptide in the treatment of chronic hepatitis B. Shijie Huaren Xiaohua Zazhi. 2001;9:388-391. |
| 15. | Chen XS, Wang GJ, Cai X, Yu HY, Hu YP. Inhibition of hepatitis B virus by oxymatrine in vivo. World J Gastroenterol. 2001;7:49-52. [PubMed] |
| 16. | Huang ZH, Zhuang H, Lu S, Guo RH, Xu GM, Cai J, Zhu WF. Humoral and cellular immunogenecity of DNA vaccine based on hepatitis B core gene in rhesus monkeys. World J Gastroenterol. 2001;7:102-106. [PubMed] |
| 17. | Fang JN, Jin CJ, Cui LH, Quan ZY, Choi BY, Ki M, Park HB. A comparative study on serologic profiles of virus hepatitis B. World J Gastroenterol. 2001;7:107-110. [PubMed] |
| 18. | Guo SP, Wang WL, Zhai YQ, Zhao YL. Expression of nuclear factor-kappa B in hepatocellular carcinoma and its relation with the X protein of hepatitis B virus. World J Gastroenterol. 2001;7:340-344. [PubMed] |
| 19. | Zhuang L, You J, Tang BZ, Ding SY, Yan KH, Peng D, Zhang YM, Zhang L. Preliminary results of Thymosin-a1 versus interferon-alpha-treatment in patients with HBeAg negative and serum HBV DNA positive chronic hepatitis B. World J Gastroenterol. 2001;7:407-410. [PubMed] |
| 20. | Wang JP, Li XH, Zhu Y, Wang AL, Lian JQ, Jia ZS, Xie YM. Detection of serum sIL-2R, IL-6, IL-8, TNF-α and lymphocytes subsets, mIL-2R in patients with chronic hepatitis B. Shijie Huaren Xiaohua Zazhi. 2000;8:763-766. |
| 21. | Zhao LS, Qin S, Zhou TY, Tang H, Liu L, Lei BJ. DNA-based vaccination induces humoral and cellular immune responses against hepatitis B virus surface antigen in mice without activation of C-myc. World J Gastroenterol. 2000;6:239-243. [PubMed] |
| 22. | Wang Y, Liu H, Zhou Q, Li X. Analysis of point mutation in site 1896 of HBV precore and its detection in the tissues and serum of HCC patients. World J Gastroenterol. 2000;6:395-397. [PubMed] |
| 23. | Li Y, Su JJ, Qin LL, Yang C, Luo D, Ban KC, Kensler T, Roebuck B. Chemopreventive effect of oltipraz on AFB (1)-induced hepatocarcinogenesis in tree shrew model. World J Gastroenterol. 2000;6:647-650. [PubMed] |
| 24. | Cheng ML, Lu YY, Wu J, Luo TY, Huang KF, Ding YS, Liu RC, Li J, Li Z. Three-year follow-up study on hepatic fibrosis due to chronic hepatitis B treated by interferon-α-1b and traditional medicine preparation. World J Gastroenterol. 2000;6:81. |
| 25. | Zheng W, Tan H, Song SB, Lu HY, Wang Y, Yu YX, Yin R. The construction and expression of a fusion protein consisting of anti-HBsAg antibody fragment Fab and interferon-a in E. coli. World J Gastroenterol. 2000;6:83. |
| 26. | Li WB, Yao ZQ, Zhou YX, Feng ZH. Studies on immunization with HBV gene vaccine plus HBsAg protein in mice. Shijie Huaren Xiaohua Zazhi. 1999;7:188-190. |
| 27. | Du DW, Zhou YX, Feng ZH, Li GY, Yao ZQ. Study on immunization of anti-subcutaneous transplanting tumor induced by gene vaccine. Shijie Huaren Xiaohua Zazhi. 1999;7:955-957. |
| 28. | Du DW, Zhou YX, Feng ZH, Yao ZQ, Li GY. Immune responses to interleukin 12 and hepatitis B gene vaccine in H2 d mice. Shijie Huaren Xiaohua Zazhi. 2000;8:128-130. |
| 29. | Wang QC, Zhou YX, Yao ZQ, Feng ZH. Effects of DNA vector constructs and different genes on the induction of immune responses by HBV DNA based vaccine. Shijie Huaren Xiaohua Zazhi. 2000;8:289-291. |
| 30. | Huang ZH, Zhuang H, Lu S, Guo RH, Xu GM, Cai J, Zhu WF. Humoral and cellular immunogenecity of DNA vaccine based on hepatitis B core gene in rhesus monkeys. World J Gastroenterol. 2001;7:102-106. [PubMed] |
| 31. | Liu HB, Meng ZD, Ma JC, Han CQ, Zhang YL, Xing ZC, Zhang YW, Liu YZ, Cao HL. A 12-year cohort study on the efficacy of plasma-derived hepatitis B vaccine in rural newborns. World J Gastroenterol. 2000;6:381-383. [PubMed] |
| 32. | Li H, Wang L, Wang SS, Gong J, Zeng XJ, Li RC, Nong Y, Huang YK, Chen XR, Huang ZN. Research on optimal immunization strategies for hepatitis B in different endemic areas in China. World J Gastroenterol. 2000;6:392-394. [PubMed] |
| 33. | Zeng XJ, Yang GH, Liao SS, Chen AP, Tan J, Huang ZJ, Li H. Survey of coverage, strategy and cost of hepatitis B vaccination in rural and urban areas of China. World J Gastroenterol. 1999;5:320-323. [PubMed] |
| 34. | Li H, Li RC, Liao SS, Yang JY, Zeng XJ, Wang SS. Persistence of hepatitis B vaccine immune protection and response to hepatitis B booster immunization. World J Gastroenterol. 1998;4:493-496. [PubMed] |
| 35. | Liao SS, Li RC, Li H, Yang JY, Zeng XJ, Gong J, Wang SS, Li YP, Zhang KL. Long-term efficacy of plasma-derived hepatitis B vaccine among Chinese children: A 12-year follow-up study. World J Gastroenterol. 1999;5:165-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Kong LB, Gao DS, Mi XQ, Wang FL. The relationship between non-and-hyporesponders to hepatitis B vaccine and their serum interleukine-2 or interleukine 6 levels. World J Gastroenterol. 2000;6:31. |
| 37. | Polakos NK, Drane D, Cox J, Ng P, Selby MJ, Chien D, O'Hagan DT, Houghton M, Paliard X. Characterization of hepatitis C virus core-specific immune responses primed in rhesus macaques by a nonclassical ISCOM vaccine. J immunol. 2001;166:3589-3598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Rimmelzwaan GF, Nieuwkoop N, Brandenburg A, Sutter G, Beyer WE, Maher D, Bates J, Osterhaus AD. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine. 2000;19:1180-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Voeten JT, Rimmelzwaan GF, Nieuwkoop NJ, Lövgren-Bengtsson K, Osterhaus AD. Introduction of the haemagglutinin transmembrane region in the influenza virus matrix protein facilitates its incorporation into ISCOM and activation of specific CD8 (+) cytotoxic T lymphocytes. Vaccine. 2000;19:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Wyde PR, Stittelaar KJ, Osterhaus AD, Guzman E, Gilbert BE. Use of cotton rats for preclinical evaluation of measles vaccines. Vaccine. 2000;19:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Wilson AD, Lövgren-Bengtsson K, Villacres-Ericsson M, Morein B, Morgan AJ. The major Epstein-Barr virus (EBV) envelope glycoprotein gp340 when incorporated into Iscoms primes cytotoxic T-cell responses directed against EBV lymphoblastoid cell lines. Vaccine. 1999;17:1282-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Mohamedi SA, Brewer JM, Alexander J, Heath AW, Jennings R. Antibody responses, cytokine levels and protection of mice immunised with HSV-2 antigens formulated into NISV or ISCOM delivery systems. Vaccine. 2000;18:2083-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Heeney J, Akerblom L, Barnett S, Bogers W, Davis D, Fuller D, Koopman G, Lehner T, Mooij P, Morein B. HIV-1 vaccine-induced immune responses which correlate with protection from SHIV infection: compiled preclinical efficacy data from trials with ten different HIV-1 vaccine candidates. Immunol Lett. 1999;66:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Verschoor EJ, Mooij P, Oostermeijer H, van der Kolk M, ten Haaft P, Verstrepen B, Sun Y, Morein B, Akerblom L, Fuller DH. Comparison of immunity generated by nucleic acid-, MF59-, and ISCOM-formulated human immunodeficiency virus type 1 vaccines in Rhesus macaques: evidence for viral clearance. J Virol. 1999;73:3292-3300. [PubMed] |
| 45. | Rytting AS, Akerblom L, Albert J, Unge T, Björling E, Al-Khalili L, Gronowitz JS, Källander CF. Monoclonal antibodies to native HIV type 1 reverse transcriptase and their interaction with enzymes from different subtypes. AIDS Res Hum Retroviruses. 2000;16:1281-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Oscherwitz J, Zeigler ME, Gribbin TE, Cease KB. A V3 loop haptenic peptide sequence, when tandemly repeated, enhances immunogenicity by facilitating helper T-cell responses to a covalently linked carrier protein. Vaccine. 1999;17:2392-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Rimmelzwaan GF, Baars M, van Beek R, de Lijster P, de Jong JC, Claas EC, Osterhaus AD. Influenza virus subtype cross-reactivities of haemagglutination inhibiting and virus neutralising serum antibodies induced by infection or vaccination with an ISCOM-based vaccine. Vaccine. 1999;17:2512-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Ennis FA, Cruz J, Jameson J, Klein M, Burt D, Thipphawong J. Augmentation of human influenza A virus-specific cytotoxic T lymphocyte memory by influenza vaccine and adjuvanted carriers (ISCOMS). Virology. 1999;259:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Rimmelzwaan GF, Claas EC, van Amerongen G, de Jong JC, Osterhaus AD. ISCOM vaccine induced protection against a lethal challenge with a human H5N1 influenza virus. Vaccine. 1999;17:1355-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Simms JR, Heath AW, Jennings R. Use of herpes simplex virus (HSV) type 1 ISCOMS 703 vaccine for prophylactic and therapeutic treatment of primary and recurrent HSV-2 infection in guinea pigs. J Infect Dis. 2000;181:1240-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Stittelaar KJ, Boes J, Kersten GF, Spiekstra A, Mulder PG, de Vries P, Roholl PJ, Dalsgaard K, van den Dobbelsteen G, van Alphen L. In vivo antibody response and in vitro CTL activation induced by selected measles vaccine candidates, prepared with purified Quil A components. Vaccine. 2000;18:2482-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |