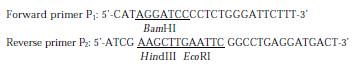Published online Apr 15, 2002. doi: 10.3748/wjg.v8.i2.276
Revised: October 22, 2001
Accepted: October 29, 2001
Published online: April 15, 2002
AIM: To establish a convenient immunoassay method based on recombinant antigen preS1(21-119 aa) to detect anti-preS1 antibodies and evaluate the clinical significance of antibodies in hepatitis B.
METHODS: The expression plasmid pET-28a-preS1 was constructed, and a large quantity of preS1(21-119 aa) fragment of the large HBsAg protein was obtained. The preS1 fragment purified by Ni2+-IDA affinity chromatography was used as coated antigen to establish the indirect ELISA based on streptavidin-biotin system for detection of the anti-preS1 antibodies in sera from HBV-infected patients. For follow-up study, serial sera were collected during the clinical course of 21 HBV-infected patients and anti-preS1 antibodies, preS1 antigen, HBV-DNA and other serological HBV markers were analyzed.
RESULTS: preS1(21-119 aa) fragment was highly expressed from the plasmid pET-28a-preS1 in a soluble form in E. coli (30 mg•L⁻¹), and easily purified to high purity over 90% by one step of Ni2+-IDA-sepharose 6B affinity chromatography. The purity and antigenicity of the purified preS1(21-119 aa) protein was determined by 150 g•L⁻¹ SDS-PAGE, Western blot and a direct ELISA. Recombinant preS1(21-119 aa) protein was successfully applied in the immunoassay which could sensitively detect the anti-preS1 antibodies in serum specimens of acute or chronic hepatitis B patients. Results showed that more than half of 19 acute hepatitis B patients produced anti-preS1 antibodies during recovery of the disease, however, the response was only found in a few of chronic patients. In the clinical follow-up study of 11 patients with anti-preS1 positive serological profile, HBsAg and HBV-DNA clearance occurred in 6 of 10 acute hepatitis B patients in 5-6 mo, and seroconversion of HBeAg and disappearance of HBV-DNA occurred in 1 chronic patients treated with lavumidine, a antiviral agent.
CONCLUSION: The high-purity preS1(21-119 aa) coated antigen was successfully prepared by gene expression and affinity chromatography. Using this antigen, a conveniently detective system of anti-preS1 antibodies in sera was established. Preliminarily clinical trial the occurrence of anti-preS1 antibodies in acute hepatitis B patients suggests the clearance of HBV from serum in a short-term time, and anti-preS1 positive in chronic patients means health improvement or recovery from the disease.
- Citation: Wei J, Wang YQ, Lu ZM, Li GD, Wang Y, Zhang ZC. Detection of anti-preS1 antibodies for recovery of hepatitis B patients by immunoassay. World J Gastroenterol 2002; 8(2): 276-281
- URL: https://www.wjgnet.com/1007-9327/full/v8/i2/276.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i2.276
Human hepatitis B virus (HBV) is a small enveloped DNA virus which causes acute and chronic hepatitis in humans[1]. Worldwide, the number of infected persons is predicted to reach 400 million during 2000. Areas with high prevalence of HBV include China[2,3], Southeast Asia and Africa, where approximately 10% of the population are chronic carriers[4]. The envelope of HBV contains three related proteins, encoded by the S open reading frame (ORF) of the viral genome, composed of three regions: preS1, preS2 and S. The major protein, termed small HBs protein (SHBs), is encoded by the S gene, whereas the two minor envelop proteins, termed the middle protein (MHBs) and the large protein (LHBs), are encoded by the preS2+S and preS1 + preS2+S region, respectively[5]. The preS1 region contains epitopes that elicit immune responses at the B-cell and T-cell level over a broader range of MHC haplotypes than those on the preS2 and S protein[6-8]. The preS1 domain also contains potential viral attachment sites to hepatocytes[9] and elicit antibodies capable of neutralizing HBV in the chimpanzee[10].
The preS1 epitopes has been extensively analyzed using synthetic peptides, anti-peptide sera and anti-preS1 monoclonal antibodies. B cell epitopes have been mapped to residues 27-35aa, 72-78aa, 32-47aa, 41-53aa, 94-105aa and 106-117aa in the preS1 region[11-13]; and T cell epitopes mainly located in residues 12-21aa, 21-30aa, 29-48aa and 94-117aa of the preS1 region[13,14]. These findings explain why the preS1 region has good immunogenicity and can easily elicit the anti-preS1 responses[15]. Additionally, nearly all preS1 epitopes concentrate on residues 21-119 aa in the preS1 region (ad subtypes of HBV LHBs carry a 119aa-residue preS1, and HBV in most Chinese belong to adr subtype; 8 residues on N-terminus of preS1 are absent in ay subtype). Because the preS1 region locates on the outside of the mature virion and has many overlapping epitopes, anti-preS1 immune response occurred early in the course of the disease and is involved in the clearance and neutralization of HBV. As anti-preS1 antibodies have been mainly detected early during acute hepatitis B[16,17], the anti-preS1 antibodies could represent an early serological marker for HBV clearance[18]. About 1/2 of subjects in the convalescence phase of acute hepatitis B were serological positive for anti-preS1 antibodies, whereas persistence of preS1 antigen and lack of corresponding antibodies have been the predict of the evolution of a chronic course of disease[19]. In this study, preS1(21-119 aa) peptide was chosen and highly expressed in a soluble form. Using this protein as the antigen, an effective enzyme-linked immunosorbent assay was established. The sensitive test can provide a basis for monitoring anti-preS1 in sera of patients with hepatitis B and offers a prognostic implication for patients.
Plasmids, E. coli strain The plasmid pADR-1 containing HBV genome (adr subtype) was constructed by Wu et al[20]. Expression vector pET-28a (+) was purchased from Novagen. E. coli strain BL21(DE3)plysS was used for the production of the preS1(21-119 aa) peptide.
Enzymes and Reagents Restriction enzymes and T4 DNA ligase were purchased from New England Biolabs, Boehringer Mannheim and GIBCO BRL. DNA sequence kit was from USB. Agrose and LMP agarose were from GIBCO BRL. Acrylamide, bisacrylamide, isopropylthio-β-D-galactoside (IPTG), imidazole and iminodiacetic acid (IDA)-Sepharose 6B, biotinamidocaproate-N-hydroxy-succinimide ester (BNSE) and 3,3',5,5'-tetramethylbenzidine (TMB) were from Sigma. Protein A was from Pharmacia. Diaminobenzidine (DAB) and streptavidin-horseradish peroxidase (HRP) conjugate were from Sino-American Biotech. Anti-preS1 (21-47aa) monoclonal antibody (mAb) 125E11 and 125E11 conjugated with HRP were made in our lab[21]. Tetrabutyl ammoniumborohydride (TBABH) was from Fluka.
Serum specimens and kits for HBV markers Serum specimens were collected from patients admitted to the Rui-Jin Hospital with clinical and biochemical evidence of hepatitis B. As a control, sera of healthy persons with normal liver function and without any markers of HBV infection were used. HBsAg and anti-HBs, HBeAg and anti-HBe, anti-HBc were determined by commercially available ELISA kits from Abbott laboratories. PreS1 protein were detected by a double mAb sandwich ELISA[22]. HBV-DNA level in serum samples was assayed by HBV PCR fluorogence diagnostic kit from PJ (Shenzhen, China).
Construction of expression plasmid containing preS1(21-119 aa) DNA sequence.
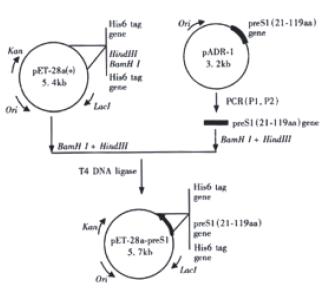
Figure 1 depicts the steps leading to the final construction of the expression plasmid pET-28a-preS1 which expresses a fusion protein containing preS1(21-119 aa) peptide with His6 tags. PCR primers were designed as follows:
Math 1
Preparation of plasmid DNA, PCR, digestion of DNA with restriction enzymes, agarose gel electrophoresis, recovery and purification of DNA fragment, ligation of terminus created by restriction enzymes and transformation of competent E. coli were performed according to Sambrook[23].
Cell culturing and harvesting E. coli strain BL21(DE3)plysS cells harboring the expression plasmid pET-28a-preS1 were inoculated into LB medium containing kanamycin (50 mg•L⁻¹). The seed culture was inoculated into 100-fold volume of fresh LB medium (kanamycin, 50 mg•L⁻¹) and cultured at 37 °C up to 0.4-0.6 of A600. To this culture IPTG was added to give a final concentration of 0.2 mmol•L⁻¹, and incubation was continued at 37 °C for 6 h or 22 °C for 24 h. The induced cells, as well as the uninduced cells, were harvested by centrifugation, and cell lysates were analyzed by 150 g•L⁻¹ SDS-PAGE.
Western analysis of the preS1(21-119 aa) fusion protein After SDS-PAGE, proteins were transferred to nitrocellulose membrane (Schleicher & Schuell), and the membrane was incubated with a murine monoclonal antibody 125E11 (1:1000, volume ratio), which recognizes preS1 (21-47aa) fragment within the preS1 region, followed by goat anti-mouse IgG peroxidase conjugate (1:1000, volume ratio). After washing, substrate solution containing DAB (0.5 g•L⁻¹) and H2O2 (0.02 mL•L⁻¹) was added to have 125E11 specific binding protein bands visualized.
Purification of the preS1(21-119 aa) fusion protein In order to characterize whether the expressed fusion protein is soluble or insoluble, the cell pellet from 100 mL culture was resuspended in 4 mL buffer A (20 mmol•L⁻¹ Tris-HCl pH7.9, 0.5 mol•L⁻¹ NaCl, 100 g•L⁻¹ glycerol, 1 mmol•L⁻¹ PMSF, 25 mmol•L⁻¹ imidazole), and sonicated in ice bath. Then the sonicate was centrifuged at 18000 r·min for 15 min, and the resulting supernatant was concentrated to 2 mL and the pellet was resuspended in 2 mL buffer A as the insoluble protein sample. The soluble and insoluble samples were subjected to 150 g•L⁻¹ SDS-PAGE and Western blot analysis.
2 mL supernatant was applied to 2 mL Ni2+ -IDA sepharose 6B column (2 mL) preequilibraed with buffer A at 4 °C. The column was washed with 30 mL buffer B (20 mmol•L⁻¹ Tri-HCl pH7.9, 0.5 mol•L⁻¹ NaCl, 100 g•L⁻¹ glycerol, 1 mmol•L⁻¹ PMSF, 45 mmol•L⁻¹ imidazole), and the bound protein was eluted with 4 mL buffer C (20 mmol•L⁻¹ Tri-HCl pH7.9, 0.5 mol•L⁻¹ NaCl, 100 g•L⁻¹ glycerol, 1 mmol•L⁻¹ PMSF, 100 mmol•L⁻¹ imidazole) and the elution samples were collected[24,25]. The fractions enriched for preS1(21-119 aa)-His6 tag fusion protein was identified by 150 g•L⁻¹ SDS-PAGE and Western blot analysis.
Biotin labeling of protein A The procedure is essentially the same as that described by Kittigul et al[26]. Protein A (2.0 g•L⁻¹) is dialyzed against 0.01 mol•L⁻¹ NaHCO3 at 4 °C. After dialysis, 1 mL of the protein A solution was mixed with 120 μL of BNSE (1.0 g•L⁻¹ in dimethyl sulfoxide). The mixture was incubated at 4 °C overnight and dialyzed overnight against PBS at 4 °Cwith several changes of PBS.
Antigenicity analysis of preS1(21-119 aa) domain of the fusion protein The antigenicity of preS1(21-119 aa) fusion protein was analyzed by a direct ELISA. The microtiter plate was coated with preS1(21-119 aa) fusion protein from 10 to 2500 ng/well, and mAb 125E11 labeled with HRP was added to each well and incubated at 37 °C for 1 h. The plate was washed, and substrate solution containing TMB (7.5 g•L⁻¹) and H2O2 (0.3 mL•L⁻¹) was added to each well to develope a color change, then the reaction was stopped with 1.0 mol•L⁻¹ H2SO4 and A (absorbance) value was measured at 450 nm in an ELISA reader (Labsystem Multiscan, Finland).
Establishment of indirect ELISA for detecting anti-preS1 antibodies in serum The optimal dilutions of the reagents are determined by checkerboard titration. Mirotiter plates (Nunc, Denmark) were coated by incubation overnight at 4 °C with 2 mg•L⁻¹ preS1(21-119 aa) protein in 100 μL volume of per well in carbonate buffer (15 mmol•L⁻¹ Na2CO3, 35 mmol•L⁻¹ NaHCO3, pH9.6). The microplates were then washed three times with a washing solution (20 mmol•L⁻¹ Tris-HCl pH7.4, 0.5 mL•L⁻¹Tween-20), and postcoated with 5 g•L⁻¹ bovine serum albumin (BSA) in PB (2.7 mmol•L⁻¹ KCl, 0.5 mmol•L⁻¹ KH2PO4, 6.5 mmol•L⁻¹ Na2HPO4, pH7.5) for 1 h at 37 °C. After washing, 100 μL diluted serum samples [1:30 in PBFST (100 mL•L⁻¹ fetal calf serum (FCS), 0.5 mol•L⁻¹ NaCl and 0.5 g•L⁻¹ Tween-20 in PB] were added and incubated for 2 h at 37 °C. After washing, 100 μL biotin labeled protein A solution (2 mg•L⁻¹ in PBFST) diluted in PBFST was dispensed into wells, and the plates were incubated for 1 h at 37 °C. After washing 4 times, streptavidin-HRP conjugate diluted in PBFST was added into each well of the plates, and the plates were incubated for 1 h at 37 °C. Finally, after washing, 100 μL of the substrate mixture (1.0 mmol•L⁻¹ TMB, 0.2 mmol•L⁻¹ TBABH, 0.2 mol•L⁻¹ potassium citrate and 0.5 mL•L⁻¹ H2O2)[27] was added and incubated for 20 min at 37 °C. The reaction was stopped by the addition of 50 μL of 1.0 mol•L⁻¹ H2SO4. The optical density was measured at 450 nm (A450) with the ELISA reader. To determine cut-off value for the established ELISA, A450 value of negative control for anti-preS1 antibodies was evaluated by testing a serum mixture from 100 normal individuals. The cut-off value was defined as 2.1 folds of mean of negative control. Specimens were considered to be positive when the obsorbance value exceeded the cut-off value.
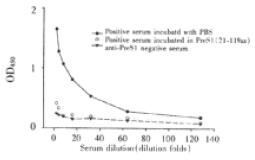
Specificity of established ELISA for detection of anti-preS1 antibodies Twenty-five μL anti-preS1 antibodies positive serum with the same volume of 0.15 g•L⁻¹ preS1(21-119 aa) protein or PBS was incubated at 37 °C, and mixture was stepwise diluted and added to wells coated by preS1(21-119 aa), and then indirect ELISA was performed. A parallel test with normal human serum was also performed in the same experiment.
Follow-up study The serum specimens from 11 anti-preS1 positive patients and 10 anti-preS1 negative patients with hepatitis B were collected at monthly intervals and their HBV markers[28], including HBV-DNA[29-31], HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HBc, preS1[32] and anti-preS1 were analyzed.
PreS1 (21-119 aa) gene fragment was synthesized by PCR using the primers P1, P2 and the plasmid pADR-1 containing HBV genome (adr subtype) as the template. The PCR product was digested with BamH Ι and Hind III and then subcloned into the BamHΙ-Hind III sites of pET-28a (+) to yield expression plasmid pET-28a-preS1 (Figure 1). Two His tag fragments which coded a stretch of six histamine residues located in both terminus of inserted fragment, respectively. This made it easy to purify preS1(21-119 aa) fusion protein by Ni2+-IDA-sepharose 6B affinity chromatography. DNA sequence analysis confirmed that the expression plasmid coded the correct nucleotide sequence. The nucleotide sequence and deduced amino acid sequence of the preS1(21-119 aa) region gene fragment inserted in the pET-28a-preS1 is shown in Figure 2.
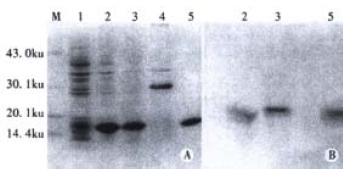
In order to express the preS1(21-119 aa) fusion protein, the expression plasmid pET-28a-preS1 was transformed into E. coli BL21(DE3)plysS, and the cells were cultured and induced by IPTG. The expressive levels were determined by SDS-PAGE and Western blot analysis of the cell lysates. As show in lane 2 of Figure 3, more than 30% of total stainable proteins was about 17000 fusion protein. The analysis of solubility showed that about 80% of the fusion protein was soluble (lane 3 of Figure 3). Thus, the soluble form was convenient for the followed purification and clinical application.
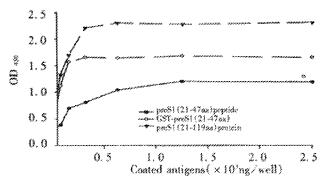
The fusion protein was purified by affinity chromatography on Ni2+-IDA sepharose 6B column as described in Materials and Methods. The purity of purified fusion proteins was evaluated to be over 90% (Lane 5, Figure 3). In order to analyze antigenicity of the fusion proteins, microtiter plates were coated with stepwise diluted solutions (from 2500 ng to 10 ng per well) of purified preS1(21-119 aa) protein, GST-preS1 (21-47aa) or artificially synthesized preS1 (21-47aa) peptide. Direct ELISA showed that they all reacted well with the preS1-specific mAb 125E11 in a dose-dependent manner, meanwhile the antigenicity of preS1(21-119 aa) protein was better than GST-preS1 (21-47aa) and much better than synthesized preS1 (21-47aa) peptide (Figure 4).
In order to observe specificity of established indirect ELISA, anti-preS1 antibodies positive serum (confirmed by Western Blotting) was preincubated with preS1(21-119 aa) fusion protein and used to test. The result showed in a drop of the extinction to a value close to the negative control, thus demonstrating specificity of the method (Figure 5).
In order to inspect established indirect ELISA, 192 serum specimens were collected from patients admitted to the Rui-Jin Hospital, Shanghai Second Medical University. Among them, there were 92 serum specimens with clinical and biochemical evidence of hepatitis B; and 100 sera of individuals without any markers of HBV infection and with normal liver function were assigned as negative controls. Additionally, 3 sera known to contain antibodies against preS1(21-119 aa) with different level tested by Western analysis were used as positive controls. Through the indirect ELISA based on recombinant preS1(21-119 aa) fusion protein, anti-preS1 antibodies were detected in none of 100 HBV negative controls. In HBsAg positive individuals (Table 1), anti-preS1 antibodies were noted in more than half of patients with acute hepatitis before or after recovery, but only found in a few of patients with chronic hepatitis, and the level of anti-preS1 antibodies had significant difference between acute hepatitis patients and chronic hepatitis patients (t test, P < 0.01).
| Subjects | n | anti-preS1 Ab (+) | positive/% |
| Acute hepatitis | |||
| Before recovery (HBsAg+) | 16 | 10 | 62.5 |
| After recovery (HBsAg-) | 17 | 9 | 52.9 |
| Chronic carriers | |||
| Healthy chronic carriers | 30 | 1 | 3.3 |
| Chronic hepatitis | 29 | 2 | 6.9 |
| Total | 92 | 22 | 23.9 |
| Control (negative forall HBV markers) | 100 | 0 | 0 |
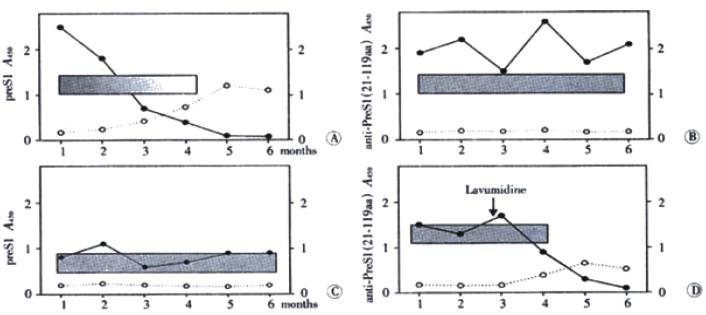
Diagnosed by clinical symptoms and serological profile, the inpatients for follow-up study were assigned to 10 acute hepatitis patients and 11 chronic hepatitis patients. Sera from 21 hepatitis B inpatients were collected at monthly intervals in half a year and anti-preS1 antibodies were detected by indirect ELISA. In Figure 6A, a typical profile of an acute episode of hepatitis B followed by seroconversion to anti-preS1 are presented. HBV-DNA and preS1 antigen were detectable simultaneously in the acute phase of the disease. Anti-preS1 developed early in infection, still in the presence of low level of preS1 antigen, and climbed the highest with disappearance of HBV-DNA and preS1 antigen. The results of detection of the other serological markers showed that anti-HBe and antiBs in sera of patients was followed by appearance of anti-preS1 antibodies.
Ten chronic hepatitis B inpatients were divided into two groups: one group was healthy chronic carriers (Figure 6B) who were seropositive HBeAg and high level of HBV-DNA and preS1 antigen; the other group was chronic hepatitis B patients (Figure 6C) who had seropositive anti-HBe and low level of HBV-DNA and preS1 antigen during the course of the disease. During follow-up period, anti-preS1 antibodies were not found and there were no apparent improvement in both groups. Interestedly, a patient (Figure 6D) treated with lavumidine, a anti-HBV-DNA replication agent, was different from the other chronic patients. Seroconversion of preS1 antigen to anti-preS1 antibodies was observed after lavumidine treatment. Although no elimination of HBsAg was observed in this patient, the development of anti-preS1 response correlated well with improvement in health.
The PreS1 domain is found exclusively in LHBs, which is a major component of the envelope of mature virions, but it is significantly less represented in subviral particles of HBV[5]. These findings suggest that PreS1 domain is located on the out surface of the virion and thought to be involved in virus-host cell interaction[33-35]. Moreover, a sequence between amino acid residues 21 and 47 of the PreS1 domain was found to be the dominant binding site for hepatocytes[9,36-38]. PreS1 domain induces an immune responses in patients recovering from HBV infection and are considered to be essential in initiating the clearance of the infectious virus for recovery from HBV infection[19,39]. Above materials indicate that the anti-preS1 antibodies in the serum of hepatitis B patients may well be a new serological marker for clinical diagnosis of hepatitis B. Previous experimental data showed that none of important epitopes were found in preS1 (1-20aa) fragment. Therefore, in present study preS1(21-119 aa) sequence of HBV (adr subtype) envelope protein was chosen for recombinant expression and application for detection of anti-preS1 antibodies.
Under the control of inducible T7 promoter, preS1(21-119 aa) fusion protein was highly expressed in soluble form by the expression plasmid pET-28a-preS1 transfected BL21(DE3)plysS cells, and was easily purified by single step of affinity chromatography, owing to His6 tags on both terminus of preS1(21-119 aa) sequence.The antigenic properties of the recombinant preS1(21-119 aa) protein were determined by the reactivity with anti-preS1 monoclonal antibody 125E11, which recognizes a linear preS1 sequence between 21aa and 47aa[21], and it is suitable for identification of preS1 proteins containing preS1 (21-47aa) fragment.
Recently, Wei et al[40] reported that they have expressed preS1 (21-47aa) fusion proteins, which were successfully used in preliminary trial to detect anti- preS1 (21-47aa) antibody. Because there are more epitopes in preS1(21-119 aa) sequence, ELISA established in this study was much better in sensitivity compared with the assay based on preS1 (21-47aa) fusion proteins as coated antigen. In this study, streptavidin-biotin system was introduced into ELISA, and this system has been used widely to detect immunoglubin[41,42]. Protein A is known to bind IgG in human and several species of animals via the Fc portion of the IgG molecule[43]. In our experimental condition, the sensitivity and specificity of ELISA based on biotin labeled protein A were much improved than ELISA based on anti-human IgG.
Clinical follow-up results showed that appearance of anti-preS1 antibody in the course of most acute hepatitis patients could predict the clearance of HBeAg and disappearance of preS1 dominants and HBV-DNA[44,45] followed by elimination of HBsAg and seroconversion to anti-HBs. The antibodies directly against preS1 region are distinct from anti-HBs, and anti-preS1 response developed after onset of the symptom in-patients and early than other HBV-related immune-responses[46,47]. The role of anti-preS1 antibodies might be neutralization of HBsAg with preS1-coded epitopes (particularly infective HBV virions), as the antibodies were found in most cases of acute hepatitis followed by recovery[48]. Anti-preS1 antibodies were hardly observed in patients with acute hepatitis progressing to chronic disease and in chronic hepatitis patients with continuing presence of preS1 domain and seropositive of HBeAg or anti-HBe[49,50]. But anti-preS1 antibodies was detected in a few patients with chronic aggressive hepatitis undergoing treatment with antiviral agents, and the appearance of the antibodies correlated well with healthy improvement[51,52]. The apparent prognostic implications of anti-preS1 antibodies are of interest in screening for this marker in hepatitis B patients.
In conclusion, the study suggested that presence of antibodies against preS1(21-119 aa) region in serum during acute infection may indicate subsequent recovery. Through detection of anti-preS1(21-119 aa) antibodies based on biotin-labeled protein A indirect ELISA and follow-up study, it afforded some information about the state and future prognosis of hepatitis B patients. The detection system has potential to be developed to a new kit for diagnosis and prognosis of hepatitis B patients.
Edited by Wang JH and Xu XQ
| 1. | Worman HJ, Feng L, Mamiya N. Molecular biology and the diagnosis and treatment of liver diseases. World J Gastroenterol. 1998;4:185-191. [PubMed] |
| 2. | Xu KC, Wei BH, Yao XX, Zhang WD. Recent therapy for chronic hepatitis B by combined traditional Chinese and western medicine. Shijie Huaren Xiaohua Zazhi. 1999;7:970-974. |
| 3. | Wu XN. Update therapy of chonic hepatitits B in China: recent progress. China Natl J New Gastroenterol. 1996;2:65-68. |
| 4. | Yu LC, Gu CH. Mutation of hepatitis B virus and its association with liver disease. Shijie Huaren Xiaohua Zazhi. 1999;7:978-979. |
| 5. | Cho EW, Park JH, Yoo OJ, Kim KL. Translocation and accumulation of exogeneous hepatitis B virus preS surface proteins in the cell nucleus. J Cell Sci. 2001;114:1115-1123. [PubMed] |
| 6. | Pride MW, Bailey CR, Muchmore E, Thanavala Y. Evaluation of B and T-cell responses in chimpanzees immunized with Hepagene, a hepatitis B vaccine containing pre-S1, pre-S2 gene products. Vaccine. 1998;16:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Milich DR, McLachlan A, Moriarty A, Thornton GB. A single 10-residue pre-S (1) peptide can prime T cell help for antibody production to multiple epitopes within the pre-S (1), pre-S (2), and S regions of HBsAg. J immunol. 1987;138:4457-4465. [PubMed] |
| 8. | Yang JY, Hui JY, Li GD, Wang Y, Yuan HY, Li YY. Expression of the Recombinant Hepatitis B Virus Surface Antigen Carrying PreS Epitopes in Pichia pastoris. Shengwu Huaxue Yu Shengwu Wuli Xuebao (. Shanghai). 2000;32:139-144. [PubMed] |
| 9. | Bock CT, Tillmann HL, Manns MP, Trautwein C. The pre-S region determines the intracellular localization and appearance of hepatitis B virus. Hepatology. 1999;30:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Neurath AR, Seto B, Strick N. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine. 1989;7:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Park JH, Cho EW, Lee YJ, Shin SY, Kim KL. Determination of the protective effects of neutralizing anti-hepatitis B virus (HBV) immunoglobulins by epitope mapping with recombinant HBV surface-antigen proteins. Microbiol immunol. 2000;44:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Kuroki K, Floreani M, Mimms LT, Ganem D. Epitope mapping of the PreS1 domain of the hepatitis B virus large surface protein. Virology. 1990;176:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Maeng CY, Ryu CJ, Gripon P, Guguen-Guillouzo C, Hong HJ. Fine mapping of virus-neutralizing epitopes on hepatitis B virus PreS1. Virology. 2000;270:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Ferrari C, Cavalli A, Penna A, Valli A, Bertoletti A, Pedretti G, Pilli M, Vitali P, Neri TM, Giuberti T. Fine specificity of the human T-cell response to the hepatitis B virus preS1 antigen. Gastroenterology. 1992;103:255-263. [PubMed] |
| 15. | Le Seyec J, Chouteau P, Cannie I, Guguen-Guillouzo C, Gripon P. Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J Virol. 1999;73:2052-2057. [PubMed] |
| 16. | Borisova G, Borschukova O, Skrastina D, Dislers A, Ose V, Pumpens P, Grens E. Behavior of a short preS1 epitope on the surface of hepatitis B core particles. Biol Chem. 1999;380:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Coursaget P, Buisson Y, Bourdil C, Yvonnet B, Molinié C, Diop MT, Chiron JP, Bao O, Diop-Mar I. Antibody response to preS1 in hepatitis-B-virus-induced liver disease and after immunization. Res Virol. 1990;141:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Ryu CJ, Kim YK, Hur H, Kim HS, Oh JM, Kang YJ, Hong HJ. Mouse monoclonal antibodies to hepatitis B virus preS1 produced after immunization with recombinant preS1 peptide. Hybridoma. 2000;19:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Prange R, Werr M. DNA-mediated immunization to hepatitis B virus envelope proteins: preS antigen secretion enhances the humoral response. Vaccine. 1999;17:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Wu XF, Zhou YZ, Feng ZM, Li ZP, Xia SY. Cloning and restriction mapping of human HBV genome serotype adr. Sci Sin B. 1983;26:954-960. [PubMed] |
| 21. | Yang HL, Jin Y, Cao HT, Xu X, Li GD, Wang Y, Zhang ZC. Affinity purification of HBV surface antigen carring preS1 region. Shengwu Huaxue Yu Shengwu Wuli Xuebao. 1996;28:412-417. |
| 22. | Yang HL, Cao HT, Li MY, Ping BF, Li GD, Wang Y, Zhang ZC. Development of the diagnostic kit to detect preS1 protein-marker of the intact Hepatitis B Virus Dane particle. Shanghai Yixue Jianyan Zazhi. 1995;10:204-206. |
| 23. | Sambrook J, Fritsch EF, Manniatis T. Molecular cloning: a laboratory manual. (2nd editor). Cold Spring Laboratory Press 1989:25-98. . |
| 24. | Park JH, Na SY, Lee HH, Lee YJ, Kim KL. Detection of pET-vector encoded, recombinant S-tagged proteins using the monoclonal antibody ATOM-2. Hybridoma. 2001;20:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Núñez E, Wei X, Delgado C, Rodríguez-Crespo I, Yélamos B, Gómez-Gutiérrez J, Peterson DL, Gavilanes F. Cloning, expression, and purification of histidine-tagged preS domains of hepatitis B virus. Protein Expr Purif. 2001;21:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Kittigul L, Temprom W, Sujirarat D, Kittigul C. Determination of tumor necrosis factor-alpha levels in dengue virus infected patients by sensitive biotin-streptavidin enzyme-linked immunosorbent assay. J Virol Methods. 2000;90:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Frey A, Meckelein B, Externest D, Schmidt MA. A stable and highly sensitive 3, 3', 5, 5'-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J immunol Methods. 2000;233:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Fang JN, Jin CJ, Cui LH, Quan ZY, Choi BY, Ki M, Park HB. A comparative study on serologic profiles of virus hepatitis B. World J Gastroenterol. 2001;7:107-110. [PubMed] |
| 29. | Zhou P, Zhang MS, Cai Q, Chen YC, Li XJ, Yu JG, Guan J, Liu CL. Detection of HBV DNA in serum of patients with HBV infection by polymerase chain reaction. Shijie Huaren Xiaohua Zazhi. 1998;6:263-264. |
| 30. | Wang PZ, Zhang ZW, Zhou YX, Bai XF. Quantitative PCR detection of HBV-DNA in patients with chronic hepatitis B and its significance. Shijie Huaren Xiaohua Zazhi. 2000;8:755-758. |
| 31. | Zheng Z, Yang SW, Xiao W, Sun P, Li XJ, Hu YQ. Evaluation of the HBV in HbsAg negative inpatients by HBV DNA measured with PCR. World J Gastroenterol. 1998;4:77. |
| 32. | Le Guillou DB, Duclos-Vallée JC, Eberle F, Capel F, Petit MA. Evaluation of an enzyme-linked immunosorbent assay for detection and quantification of hepatitis B virus PreS1 envelope antigen in serum samples: comparison with two commercial assays for monitoring hepatitis B virus DNA. J Viral Hepat. 2000;7:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Kuijpers L, Koens M, Murray-Lyon I, Coleman JC, Karayiannis P, Thomas HC, Zanetti A, Cargnel A, Harrison TJ, Zuckerman AJ. Pre-S proteins in hepatitis B. J Med Virol. 1989;28:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Chen K, Han BG, Ma XK, Zhang HQ, Meng L, Wang GH, Xia F, Song XG, Ling SG. Establishment and preliminery use of hepatitis B virus preS1/2 antigen assay. World J Gastroenterol. 1999;5:550-552. [PubMed] |
| 35. | Choi IH, Park SG, Chung JH, Kim IJ, Hong HJ. Generation of human Fab monoclonal antibodies against preS1 of hepatitis B virus using repertoire cloning. Hybridoma. 1998;17:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Neurath AR, Kent SB, Strick N, Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986;46:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 412] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 37. | Hui J, Mancini M, Li G, Wang Y, Tiollais P, Michel ML. Immunization with a plasmid encoding a modified hepatitis B surface antigen carrying the receptor binding site for hepatocytes. Vaccine. 1999;17:1711-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Ono M, Morisawa K, Nie J, Ota K, Taniguchi T, Saibara T, Onishi S. Transactivation of transforming growth factor alpha gene by hepatitis B virus preS1. Cancer Res. 1998;58:1813-1816. [PubMed] |
| 39. | Hu YP, Yao YC, Li JX, Wang XM, Li H, Wang ZH, Lei ZH. The cloning of 3'-truncated preS/S gene from HBV genomic DNA and its expression in transgenic mice. World J Gastroenterol. 2000;6:734-737. [PubMed] |
| 40. | Wei J, Liu XJ, Li GD, Wang Y, Zhang ZC, Wang YQ, Lu ZM. Expression, Purification and Preliminary Clinical Use of Recombinant HBsAg GST-PreS1 (21--47 aa) Fusion Proteins. Shengwu Huaxue Yu Shengwu Wuli Xuebao (. Shanghai). 2001;33:379-385. [PubMed] |
| 41. | Bolton J, Sanders J, Oda Y, Chapman C, Konno R, Furmaniak J, Rees Smith B. Measurement of thyroid-stimulating hormone receptor autoantibodies by ELISA. Clin Chem. 1999;45:2285-2287. [PubMed] |
| 42. | Santora KE, Nelson SA, Lewis KA, LaRochelle WJ. Avidin- or streptavidin-biotin as a highly sensitive method to stain total protein on membranes. Mol Biotechnol. 2000;15:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Praul CA, Brubaker KD, Leach RM, Gay CV. Detection of endogenous biotin-containing proteins in bone and cartilage cells with streptavidin systems. Biochem Biophys Res Commun. 1998;247:312-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Tang RX, Gao FG, Zeng LY, Wang YW, Wang YL. Detection of HBV DNA and its existence status in liver tissues and peripheral blood lymphocytes from chronic hepatitis B patients. World J Gastroenterol. 1999;5:359-361. [PubMed] |
| 45. | Reshetnyak VI, Sharafanova TI, Ilchenko LU, Golovanova EV, Poroshenko GG. Peripheral blood lymphocytes DNA in patients with chronic liver diseases. World J Gastroenterol. 2001;7:235-237. [PubMed] |
| 46. | Heermann KH, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich WH. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984;52:396-402. [PubMed] |
| 47. | Yang JY, Jin J, Kong YY, Wei J, Zhang ZC, Li GD, Wang Y, Yuan HY, Li YY. Purification and Characterization of Recombinant Hepatitis B Virus Surface Antigen SS1 Expressed in Pichia pastoris. Shengwu Huaxue Yu Shengwu Wuli Xuebao (. Shanghai). 2000;32:503-508. [PubMed] |
| 48. | Milich DR, Thornton GB, Neurath AR, Kent SB, Michel ML, Tiollais P, Chisari FV. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985;228:1195-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 244] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Mimms LT, Floreani M, Tyner J, Whitters E, Rosenlof R, Wray L, Goetze A, Sarin V, Eble K. Discrimination of hepatitis B virus (HBV) subtypes using monoclonal antibodies to the PreS1 and PreS2 domains of the viral envelope. Virology. 1990;176:604-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Pontisso P, Ruvoletto MG, Gerlich WH, Heermann KH, Bardini R, Alberti A. Identification of an attachment site for human liver plasma membranes on hepatitis B virus particles. Virology. 1989;173:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 116] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Klinkert MQ, Theilmann L, Pfaff E, Schaller H. Pre-S1 antigens and antibodies early in the course of acute hepatitis B virus infection. J Virol. 1986;58:522-525. [PubMed] |
| 52. | Milich DR. Genetic and molecular basis for T- and B-cell recognition of hepatitis B viral antigens. Immunol Rev. 1987;99:71-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |