MATERIALS AND METHODS
Selection of samples
20 liver tissue samples were collected from the patients of liver cancer hospitalized in Department of Pathology of Affiliated Hospital to Binzhou Medical College as the liver cancer group (12 from males, 8 from females). Their tissues adjacent to the cancer were used as the cancer adjacent tissue group. The preparation of tissue block and slices: the samples obtained surgically were fixed with 10% formalin, dehydrated by conventionality, embedded by paraffin, cut into slices 5 μm thick. One slice for each sample was stained by HE; it was used for pathological identification.
In vivo detection of apoptosis
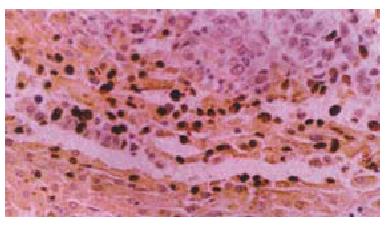
TUNEL staining was used to detect DNA degradation in situ in the relatively late stage of apoptosis. Apoptotic cells were labelled by the TUNEL reaction using an in situ Cell Apoptosis Detection Kit. In situ Cell Apoptosis Detection Kits were purchased from WUHAN BOSTER Biological Technology Co, Ltd. The detailed manipulation were conducted according to introductions for users. Negative controls consists of the process except that the labelling buffer was substituted for TdT and DIG-dUTP. positive controls were hypothyroid cancer samples. Result identification: After DAB staining the cells whose nuclei were orange counted as positive. Photomicrograghs were taken with an Olympus microphoto-microscope (see Figure 1).
Figure 1 shows apoptotic cells in liver carcinomas and in cancer adjatent tissues (positive).
TUNEL × 200
Data processing
The positive rates for each group were calculated.Four visual fields were randomly selected from each tissue section and analyzed under 10 × 20 fold statistics microscope with the imaging analysis system of CMIAS. The integrated optical density (IOD) was measured, and data were expressed as means ± sem, and analyzed with t-test by software Microsoft Excel 97.P pvolume less than 0.05 was regarded as representing significant difference.
Drug preparation
Solanum lyratum Thumb is the whole plant of Solanum lyratum Thumb deadly nightfade. After extraction using ethanol, and there after using acetidine, the fat-soluble extracts of Solanum lyratum Thumb.were distilled in DMSO and then diluted in RPMI 1640 medium to different concentrations for later use.
Cell culture and drug application
Human hepatoplastoma cell line BEL-7404 was purchased from Institute of Materia Medica, Chinese Academy of Medical Science, Beijing. BEL-7404 cells were seeded at 37 °C in a humidified incubator under 5% CO2/95% air in RPMI 1640 supplemented with 200 IU/mL penicillin, 200 μg/mL streptomycin, and cultured for 24 h. This medium was replaced by another medium containing extracts of Solanum lyratum Thumb 10 μg•mL-1 、5 μg•mL-1 、2.5 μg•mL-1 respectively. The cells went on to be cultured for 24, 48, 72 h.
In vitro detection of apoptosis
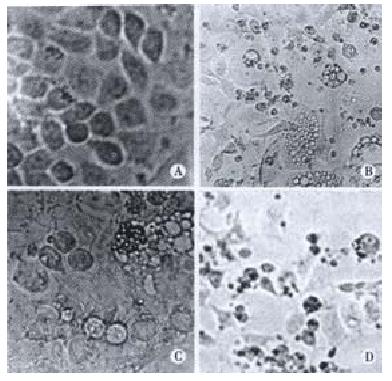
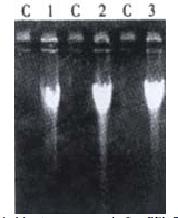
Cellular morphological observation: First the cell shapes were observed and apoptosis was detected and photomicrograghs were taken with an Olympus microphoto-microscope.DNA isolation and electrophoresis: After the cells were cultured in deferent concentrations of the drug for deferent time lengths, DNA isolation was done as follows: After they were washed twice with RPMI 1640 medium and resuspended, the cells (5 × 105 or 2 × 106 cells)were collected by centrifugation (200 × g, 10 min), and then 50 μL of cell cracking liquid was added to resuspend the cells.They were incubated at 37 °C until the lysates containing liquid became clear, and then centrifuged at 12000 r·min-1 5 min at 4 °C. DNA fragments were extracted from the supernatant with phenol-chloroform (1:1)phenol-chloroform-isoamyl alcohol (25:14:1), precipitated by addition of 2 volumes of absolute ethanol and 0.1 volume of 3 mol•L⁻¹ sodium acetate at -20 °C for 24 h, centrifugated at 12000 r·min-1 -10 °C, for 10 min.The precipitate was dissolved in 20 μL TE with 1 μL RNase and kept at 37 °C for 1 h. The pattern of DNA fragmentation was visualized by electrophoresis (at room temperature, 75 mA, 1-2 h) in 1% agarose gel containing ethidium bromide and photographed under UV light.
DISCUSSION
The term ‘apoptosis’ was introduced into modern science by Kerr et al[4] in 1972 to describe the special morphology of physiological cell death. The word itself is an ancient Greek word meaning the ‘falling off’ of leaves from a tree or petals from a flower and was originally used in the medical and philosophical writings of classical Greek and Roman times. Apoptosis or‘ programmed cell death’ represents the regulated activation of a pre-existing death program encoded in the genome. It is a highly orchestrated form of cell death and plays a central role in the control of tissue cell numbers in organs' development, homeostasis and normal functioning, such as cell proliferation and differentiation. Dysregulation of apoptosis may be involved in the pathogenesis of human diseases[5].
There are two central pathways that lead to apoptosis: (1) positive induction by ligand binding to a plasma membrane receptor and (2) negative induction by loss of a suppressor activity. Each leads to activation of cysteine proteases with homology to IL-1b converting enzyme (ICE) (i.e., caspases). Positive-induction involves ligands related to TNF. The ligands are typically trimeric and bind to cell surface receptors causing aggregation (trimerization) of cell surface receptors. The intracellular portion of these receptors contains an 80 amino acid death domain (DD) that through homophilic interactions recruits adaptor proteins to form a signaling complex on the cytosolic surface of the receptor. The bringing together of the three receptors, thereby orienting the intracellular DDs, appears to be the critical feature for signaling by these receptors. The adaptor complex then recruits caspase-8; caspase-8 is activated, and the cascade of caspase-mediated disassembly proceeds[6,7]. Cytosolic Aspartate-Specific Proteases, called CASPases, are responsible for the deliberate disassembly of a cell into apoptotic bodies. Caspases are present as inactive pro-enzymes, most of which are activated by proteolytic cleavage. Caspase-8, caspase-9, and caspase-3 are situated at pivotal junctions in apoptotic pathways. Caspase-8 initiates disassembly in response to extracellular apoptosis-inducing ligands and is activated in a complex associated with the receptors' cytoplasmic death domains. Caspase-9 activates disassembly in response to agents that trigger release of cytochrome c from the mitochondria and is activated when complexed with dATP, APAF-1, and extramitochondrial cytochrome c. Caspase-3 appears to amplify caspase-8 and caspase-9 signals into full-fledged commitment to disassembly. Both caspase-8 and caspase-9 can activate caspase-3 by proteolytic cleavage and caspase-3 may then cleave vital cellular proteins or activate additional caspases by proteolytic cleavage[8-10].
Negative induction of apoptosis by loss of a suppressor activity involves the mitochondria. Release of cytochrome c from the mitochondria into the cytosol serves as a trigger to activate caspases[11]. Permeability of the outer mitochondrial membrane is essential to initiation of apoptosis through this pathway. Proteins belonging to the Bcl-2 family appear to regulate the membrane permeability to ions and possibly to cytochrome c as well. Although these proteins can themselves form channels in the membrane, the actual molecular mechanisms underlying the regulation of mitochondrial permeability and the release of solutes remain to be elucidated. The Bcl-2 family is composed of a large group of anti-apoptosis members that when overexpressed prevent apoptosis and a large group of pro-apoptosis members that when overexpressed induce apoptosis. The balance between the anti-apoptotic and pro-apoptotic Bcl-2 family members may be critical to determining whether a cell undergoes apoptosis. Thus, the suppressor activity of the anti-apoptotic Bcl-2 family appears to be negated by the pro-apoptotic members[12]. Many members of the pro-apoptotic Bcl-2 family are present in cells at levels sufficient to induce apoptosis. However, these members do not induce apoptosis because their activity is maintained in a latent form. Bax is present in the cytosol of living cells. After an appropriate signal, Bax undergoes a conformational change and moves to the mitochondrial membrane where it causes release of mitochondrial cytochrome c into the cytosol. BID is also present in the cytosol of living cells. After cleavage by caspase-8, it moves to the mitochondria where it causes release of cytochrome C possibly by altering the conformation of Bax. Similarly, BAK appears to undergo a conformational change that converts it from an inactive to an active state. Thus, understanding the molecular mechanisms responsible for regulating the Bcl-2 family activities creates the potential for pharmaceutical intervention to control apoptosis[13-16].
Liver cancinoma is one of the most common cancers. Further progress has been made in its diagnoses, treatments and the mechanism of its occurrence. The study of its apoptosis is very important[17,18]. It has been proved that occurrence of cancers is due to the loss of control of normal apoptosis and the disturbance of balance between cell apoptosis and cell proliferation[1,2]. The apoptosis related genes (bcl-2 family) is divided into two categories: Apoptotic repressor and apoptotic promoter. Bcl-2 is an important apoptotic repressor, while Bax is one of the most important apoptotic promoters. The protein it encodes can combine with Bcl-2 to form compounds which resist the action of repressing apoptosis. But it has a positive regulatory action[19-21].Recant studies indicate that the regulation of apoptosis by bcl-2 and bax is not only based on the level of either of the two regulatory proteins but also based on the ratio of them. If the ratio is high, the cells go to apoptosis[22-24]. In our experiment the in vivo apoptosis in liver cancers was detected by TUNEL technique. The result showed that IOD in the liver cancer group was lower than in the cancer adjacent tissue group, which was a significant difference (t = 2.2011, P < 0.05). Our results were consistent with that of Peng et al[25]. We found that apoptotic cells in the cancer adjacent tissue were more and there were less in the cancer. This showed that apoptosis in the cancer adjacent tissue is more active. The apoptosis may be caused by a negative induction of apoptosis. First the level of BAX can increase. Its conformations can change, and then move to the outer membrane of mitochondria thus making cytochrome c to release from mitochondria. There after CASPASE is activated, and then the cascade of CASPASE -mediated disassembly proceeds. Thus apoptosis emerges. The pathway may be blocked in liver cancers, and apoptosis slow down, so proliferation of the cells cannot balanced, and the cancers occur.
Some drugs (e.g. adriamycin, doxorubicin, propolis)can induce apoptosis, repress the division of liver cancer cells and thus treat cancers[26-28]. Tamoxifen can induce apoptosis of HepG2 hepatoblasma cells, because Tamoxifen activates Ca2+ influx pathways. After the human hepatocellular SK-HEP-1 carcinoma cells were treated with methylglyoxal bis (cyclopentylamidinohydrazone) (MGBCP), their cell morphology changed, and the cell growth rate decreased. It may induce cell apoptosis[29,30]. Human hepatoma cell line (HepG2) were treated with Sodium selenite. Selenite-induced DNA alterations in apoptosis were studied, and characteristic apoptotic morphological alterations were observed. The results clearly show that Se-induced cell death occurs predominantly in the form of apoptosis[31]. Apoptosis in human hepatoma BEL-7402 cells can be induced by Octreotide, which may be related to the mechanism of antineoplastic action of Octreotide in hepatoma[32]. It has been proved that baicalein, chlorinated fatty acids, troglitazone (an antidiabetic agent)and cisplatin could respectively induce apoptosis in hepatoma cells HepG2[33-36].Their results were similar to ours. The apoptostic characteristics, DNA fragment, cellular morphological changes emerged in the hepatoma cells treated with deferent agents.
Solanum Lyratum is one of Chinese anticancer herbals.The damage effect of Solanum Lyratum on different phases of PC chromosomes in CHO cells was studied by cell fusion technique. The result showed that obvious damage were observed in G2-PCC in cells treated with the drug at 0.5-1.0 g•mL-1 for 45 min at 37 °C. The obvious damage of PC chromosomes indicated that Solanum Lyratum may markedly block the G2 phase of cell cycle. But treatment with different doses of the drug showed no effect on the progression of cells in M phase. A study found that Chinese Herbal Mixture containing Solanum Lyratum has a stronger killing action on Human Tumor Cells BGC-823 and MCF-7 in vitro. Flowcytometric analysis showed that the drug can stop the cell cycle of BGC-823 at G1 phase and cause a significant decrease in the number of S phase cells. It was found that the drug can inhibit DNA synthesis, expressions of antioncogenes Rb, P21 and enhance the expresion of oncogene c- myc in BGC-823 cells[37]. Apoptosis of cancers is induced with the Chinese herbal medicine[38-40]. Solanum Lyratum can suppress the proliferation of hepatoma cells via G0/G1 arrest and inhibition of DNA synthesis followed by apoptosis. Arsenic trioxide has significant selective apoptosis-inducing effect on human hepatocarcinoma cells, which is regulated by several genes.Bcl-2 gene expression down-regulated, Bax and Fas gene expressions up-regulated[41]. A Chinese herb-magnolol can also inhibit the proliferation of tumor cells and activate apoptosis in vitro and in vivo[42].
In our experiment, after BEL-7404 human hepatoblasma cell line was cultured in the medium containing Solanum Lyratum extract (concentration: 2.5 μg•mL-1 、5 μg•mL-1 、10 μg•mL-1)for 24 h, the morphologic change took place in these cells, and the apoptotic bodies and DNA ladders appeared. After 48 h, 72 h they changed acutely. This indicated that Solanum Lyratum may exert an anticancer action through promoting apoptosis and resulting in the death of the liver cancer cells. it may stimulate the cells to bring out a positive gene directed apoptosis. It probably induce overexpression of gene bax and inhibit expression of gene bcl-2, making the ratio of proteins of bax/bcl-2 increase and then promoting the cell apoptosis.This may be a positive induction of apoptosis. First Solanum Lyratum as a ligand binds to cell surface receptor.It causes aggregation of cell surface receptors. The signaling complex on the cytosolic surface of the receptor forms. CASPASE is activated, and then the cascade of CASPASE -mediated disassembly proceeds.
Several genes, factors and TNF receptor family are involved in the apoptosis of liver cancer cells. In the human hepatocellular carcinoma cell line, Hep3B, an increased level of bax expression mediates apoptosis[43].It was found that bcl-2-positive cells did not show apoptosis, and P53 protein (stimulative) may regulate apoptosis in some cases of human liver tumors, the mutant P53 protein may inhibit the cancer cells going to the apoptosis, whereas c- myc, Fas and Lewisy are not relevant to apoptosis[25,44,45].Bcl-2 was focally expressed in hepatic tumor epithelium[21].Using immuno-histochemical methods, the expression of Bcl-2, an anti-apoptotic protein was studied in hepatocellular tumors of B6C3F1 mice. Normal mouse hepatocytes did not express detectable amounts of Bcl-2, whereas most diethylnitrosamine-induced tumors were positive for this protein. This explained that expression of bcl-2 might inhibit the normal proliferation of cells, and result in the occurrence of tumors[46]. In the BEL-7404 cells where apoptosis was mediated by AFP, Fas apoptosis signals and Bcl-2 survival signals from apoptosis were expressed[47]. the proapoptotic protein Bax is translocated from the cytosol to mitochondria, where a potent proapoptotic 18-kDa fragment (Bax/p18) at its N-terminus of Bax was cleaved. It promotes release of cytochrome c, a caspase-activating protein, activation of caspase-3, cleavage of poly (ADP-ribose) polymerase, and fragmentation of DNA and apoptosis. On the contrary Bcl-2 overexpression inhibited etoposide-induced calpain activation, Bax cleavage, cytochrome c release, and apoptosis[48].In human hepatoma cells, Bcl-2 may protect cells from TGF-beta-F-induced apoptosis by interfering TGF-beta generated signals leading to the production of reactive oxygen species[49]. Ethanol could induce apoptosis in liver cells, which was initiated by the intracellular Ca (2+) elevation in the cytoplasm. N-p-tosyl-l-lysine chloromethyl ketone (TLCK)-sensitive serine proteases was activated[50].
Combination of drugs can increase the pharmacotherapeutic effect on liver cancers. Co-administration of the chemosensitizer verapamil increased the antitumor efficacy of paclitaxel by up to five-fold. cyclin A and cdc2 kinase accumulated in paclitaxel-treated cells. Exposure to paclitaxel decreased [3H] thymidine incorporation into DNA in cells at 24 h but this significantly increased at 72 h, that was most likely due to DNA repair mechanisms related to cell cycle restriction[51].Glutathione-doxorubicin (GSH-DXR) effectively induced apoptosis in rat hepatoma cells (AH66) at a lower concentration than DXR.DXR and GSH-DXR induce apoptotic DNA fragmentation via caspase-3 activation, but not via caspase-1 activation, and GSH-DXR enhances the activation of caspase-3 approximately 100-fold more effectively than DXR used alone. An upstream apoptotic signal that can activate caspase-3 is activated within 6 h by treating AH66 cells with the drug[52]. Internucleosomal DNA fragmentation, a biological hallmark of apoptosis, was detected in hepatoma cells after continuous incubation with a combination of sodium butyrate and interferon-alpha (IFN-alpha) for 72 h. Sodium butyrate potently enhances the anti-tumour effect of IFN-alpha in vitro and a rational combination of these two drugs may be useful for the treatment of liver cancer[53]. After 96 h in HepG2, Hep1B, Hepa1-6 and MH1C1 hepatoma cells, combination therapy of an acyclic retinoid and tamoxifen (TAM) on proliferation and apoptosis of hepatoma cell enhanced apoptosis from a maximum of 60% after monotherapy to more than 90%. Apoptosis increased p27, bax, caspase 3 expression was accompanied by upregulation of caspase 3 and 8 activity, while the levels of p21cip/waf and bcl-2 were unchanged or decreased[54]. The combination of human peripheral blood dendritic cells (DCs) and lymphokine and phytohaemagglutininum (PHA) activated killer (LPAK) cells could induce apoptosis of BEL-7402 cells effectively, with some LPAK cells manifesting the characteristics of autophagic apoptosis[55].
Clinical resistance to chemotherapeutic drugs is a major problem in the treatment of cancers. Increased bcl-2 expression in epithelium adjacent to tumors represents a genetically variation in the morphologically normal epithelium because it occurs without the corresponding high proliferative state seen in the normal crypt-regenerative compartment. Heterogeneity may provide a mechanistic explanation for chemotherapeutic resistance in tumors with cells having high bcl-2[22]. Treatment with taxol can induce apoptosis of HepG2 thus killing liver cancer cells. DNA ladder of 200 bp emerged. In the meantime bcl-xl, or bad and bax were expressed in HepG2 cells; and the expression levels of bcl-xl and bak remained unchanged, whereas the level of bad was down-regulated. It was thought that the change of bcl-2 family proteins caused by anticancer drugs in liver cancer cells may be involved in chemoresistance[56]. A new somatostatin analogue, TT-232 has a pronounced antiproliferative effect on differentiated and dedifferentiated, drug-sensitive and multidrug-resistant hepatocellular carcinoma cell lines. TT-232 induces apoptosis at comparable levels in all these hepatoma variants, which demonstrats that the multidrug resistance of hepatomas does not correlate with a reduced susceptibility to apoptosis induction. The mechanism involved in apoptosis is functional in both drug-sensitive and resistant hepatoma variants and can be activated by the somatostatin analogue TT-232[57].
The gene therapeutics to liver cancer is a new technique. Human genes P53, B7-1, GM-CSF, IL-2 were transduced into liver cancer cells by adenovirus.The ad-multigenes can induce apoptosis and elevate sensitivity of liver cancer cells to chemotherapy drug-cisplatin[58]. After gene therapy with HSV thymidine kinase, inhibition of enhanced glucose transport in ganciclovir -treated Morris hepatoma cells increased apoptosis[59].For gene therapy of hepatocellular carcinoma (HCC), the Escherichia coli purine nucleoside phosphorylase (PNP)/fludarabine suicide gene system may be more useful than the herpes simplex virus thymidine kinase/ganciclovir (HSV-tk/GCV) system. Human HCC cells of the cell lines, HepG2 and Hep3B were transduced with PNP or HSV-tk using adenoviral vectors, followed by prodrug incubation. Both systems predominantly induced apoptosis in HepG2 and Hep3B cells. The results showed that cell line-specific differences in response to treatment with PNP/fludarabine and HSV-tk/GCV, respectively. PNP/fludarabine may be superior to HSV-tk/GCV for the treatment of human HCC because of its independence from p53 and the Fas/FasL system[60].