Published online Oct 15, 2001. doi: 10.3748/wjg.v7.i5.690
Revised: August 6, 2001
Accepted: August 12, 2001
Published online: October 15, 2001
AIM: To prepare a cancer vaccine (H22-DC) expressing high levels of costimulatory molecules based on fusions of hepatocarcinoma cells (H22) with dendritic cells (DC) of mice and to analyze the biological characteristics and induction of specific CTL activity of H22-DC.
METHODS: DCs were isolated from murine spleen by metrizamide density gradient centrifugation, purified based on its characteristics of semi-adhesion to culture plates and FcR-, and were cultured in the medium containing GM-CSF and IL-4. A large number of DC were harvested. DCs were then fused with H22 cells by PEG and the fusion cells were marked with CD11c MicroBeads. The H22-DC was sorted with Mimi MACS sorter. The techniques of cell culture, immunocytochemistry and light microscopy were also used to test the characteristics of growth and morphology of H22-DC in vitro. As the immunogen, H22-DC was inoculated subcutaneously into the right armpit of BALB/C mice, and their tumorigenicity in vivo was observed. MTT was used to test the CTL activity of murine spleen in vitro.
RESULTS: DC cells isolated and generated were CD11c+ cells with irregular shape, and highly expressed CD80, CD86 and CD54 molecules. H22 cells were CD11c- cells with spherical shape and bigger volume, and did not express CD80, CD86 and CD54 molecules. H22-DC was CD11c+ cells with bigger volume, being spherical, flat or irregular in shape, and highly expressed CD80, CD86 and CD54 molecules, too. H22-DC was able to divide and proliferate in vitro, but its activity of proliferation was significantly decreased as compared with H22 cells and its growth curve was flatter than H22 cells. After subcutaneous inoculation over 60 d, H22-DC showed no tumorigenecity in mice, which was significantly different from control groups (P < 0.01). The spleen CTL activity against H22 cells in mice implanted with fresh H22-DC was significantly higher than control groups (P < 0.01).
CONCLUSION: H22-DC could significantly stimulate the specific CTL activity of murine spleen, which suggests that the fusion cells have already obtained the function of antigen presenting of parental DC and could present H22 specific antigen which has not been identified yet, and H22-DC could induce antitumor immune response; although simply mixed H22 cells with DC could stimulate the specific CTL activity which could inhibit the growth of tumor in some degree, it could not prevent the generation of tumor. It shows that the DC vaccine is likely to become a helpful approach in immunotherapy of hepatocarcinoma.
- Citation: Zhang J, Zhang JK, Zhuo SH, Chen HB. Effect of a cancer vaccine prepared by fusions of hepatocarcinoma cells with dendritic cells. World J Gastroenterol 2001; 7(5): 690-694
- URL: https://www.wjgnet.com/1007-9327/full/v7/i5/690.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i5.690
T lymphocyte-mediated immunoresponse plays an important role in the antitumor immune response. The sensibilization, activation and proliferation of T lymphocytes depend on antigen presenting cells (APC) which was able to present corresponding antigen peptides and to provide costimulatory signals[1]. However, many tumor cells have weak immunogenicity which expressed low levels or no MHC and costimulatory molecules, so that tumor antigen can not be effectively presented. Therefore, they cannot induce effective antitumor immune response in host, and can not effectively activate specific killing mechanism. APC cancer vaccines are expected to enhance the immunogenicity of tumor cells and increase the presenting ability of antigen presenting cells as well as induce effective specific T lymphocyte mediated antitumor immune response. Dendritic cells (DC) are a kind of the most potential and professional antigen presenting cells in vivo[2-8]. DC can catch, process and present antigens, and enhance the killing activity of lymphokine[9-11], especially it can powerfully stimulate the primary immune response[12-16]. So more and more attention has been paid to the function of tumor immunotherapy of DC[17-19]. In this experiment, in order to effectively strengthen the function of antigen presenting of DC, to enhance the immunogenicity of tumor cells and to stimulate the specific CTL activity of host, dendritic cells derived from murine spleen were fused with hepatocarcinoma cells.
Male BALB/C mice, 6-8 wk old, weighing 15 g-20 g, purchased from Shanghai SIPPR/BK Experimental Animal Limited, were randomly divided into test group and control group. Mouse monoclonal antibody CD80, CD86, CD54 were purchased from Coulter Co. rmGM-CSF and rmIL-4 were obtained from R&D Co. Mini MACS (magnetic cell seperation) and CD11c (N418) microBeads were bought from Miltenyi GmbH Biotec. Metrizamide was obtained from Amresco Co. and PEG was from Sigma Co. Mouse hepatocarcinoma cell line (H22) was obtained from the Cancer Research Institute of Dalian Medical University.
Isolation of DC According to the previous METHODS[20-24] with minor modefications, DCs were isolated from murine spleen by metrizamide (145 g•L¯¹) density gradient centrifugation, purified based on its characteristics of semi-adhesion to culture plates and FcR-, and cultured in the medium containing GM-CSF and IL-4 (500 ng•L¯¹), and a large number of DC were harvested.
Cell fusion and selecting[25,26] DCs were fused with H22 cells by PEG and the fusion cells were marked with CD11c MicroBeads. The H22-DCs were sorted with Mimi MACS sorter. Fused cells were cultured in RPMI 1640 medium containing 20 mL•L¯¹ fetal bovine serum, rmGM-CSF and rmIL-4 (500 ng•L¯¹) for 2-3 wk.
Cellular morphological analysis Light microscopy and phase contrast microscopy were used to identify the morphological characteristics of H22-DC, H22 and DC.
Immunocytochemical staining for CD80, CD86 and CD54 Cells were incubated with antibodies against CD80, CD86 and CD54. Membrane proteins were detected by ABC reagen and DAB staining, and photomicrographs were taken with an Olympus microphoto-microscope.
Cell proliferation analysis in vitro DCs were added into 24-well plates at 1.25 × 104 cells per well and three wells were randomly seclected to be counted every 24 h. Then, growth curve of H22-DC was drawn accoding to their average value, using H22 as control group at the same time.
Tumorigenecity assays This experiment was conducted in 3 groups. Each group included four experimental subgroups (H22-DC, H22+DC, H22 and PBS). Immunogen (0.1 mL 1 × 1010-2 × 1010•L¯¹) of H22-DC was inoculated subcutaneously into the right armpit of H22-DC subgroup mice of each group and the same amount of H22, H22+DC and PBS were inoculated into the mice in each corresponding subgroup in the same way. The growth of tumors was observed every day and the survival time of mice was calculated. Meanwhile, mice in the second group were killed on the 14th day after implantation, and tumors were isolated and tumor weight was compared.
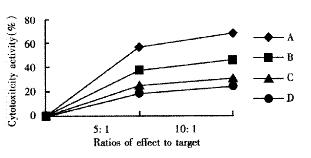
CTL activity assays The third group mice were killed for examination at the 10th day after implantation, and the spleen was separated to prepare cell suspension, then the cells were cultured in 100 mL•L¯¹ FCS-RPMI1640 medium containing the final concentration of 100 KU•L¯¹ rhIL-2 by genetic recombination at 37 °C in a saturated humidified 50 mL•L¯¹ CO2 atmosphere for 3 d. The anti-tumor experiment was conducted in four subgroups. Two ratios of effect (CTL) to target (H22) (5∶1 and 10∶1) were used in all groups. ① Group A: CTL (H22-DC subgroup) + H22; ② group B: CTL (H22+DC subgroup) + H22; ③ group C: CTL (H22 subgroup) + H22; ④ group D: CTL (PBS subgroup) + H22. In addition, T groups were only consisted of CTL as the corresponding control groups and group E was H22 control group. Culture medium of the control group only contained 100 mL•L¯¹ FCS-RPMI1640. All of these groups were cultured in 96-well culture plates and each group had 3 wells at 37 °C in a saturated humidified 50 mL•L¯¹ CO2 atmosphere for 48 h. Cytotoxity activity was determined by MTT assay as previously described[27,28]. Briefly, freshly prepared and filtered 20 μL MTT (5 g•L¯¹ in PBS) were added to each well, and the cells were continuously cultured for 4 h. Then the supernatant was removed and 150 μL DMSO was added to each well and agitated for 10 min to fully liquefy crystals, followed by reading on BIO-RAD 3550-UV type automatic ELSIA reader at 570 nm wavelength.
Statistical analysis was made using analysis of variance, if P < 0.05, the result was considered statistically significant.
DCs are irregular shaped cells with many surface membrane processes, including spiky or spherical pseudopod-like processes. They have oval or irregular-shaped nucleus with wavy movement. The cytoplasm contains rich spherical mitochondrias. Determined by immunocytochemical staining, DCs were CD80 and CD86 and CD54 positive cells with irregular shape and brown-yellow fine granules in cytoplasm (Figure 1, Figure 2, Figure 3).
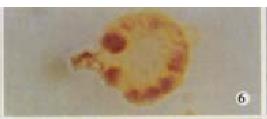
Marked with CD11c MicroBeads and sorted with Mini MACs, H22-DCs were CD11c+ cells (Figure 4, Figure 5, Figure 6), but H22 was CD11c- cells. By immunocytochemical staining, H22-DCs were CD80, CD86 and CD54 positive cells and H22 was negative cells. Cytokine of rmGM-CSF and rmIL-4 was able to induce proliferation of fusion cells and prolong their survival time. The fusion cells which were marked with CD11c MicroBeads and sorted with Mini MACs were mixed with unfused DC. If there were no rmGM-CSF and rmIL-4 in the medium, natural apoptosis would occur in DC after 10-14 d, but H22-DC would be still alive.
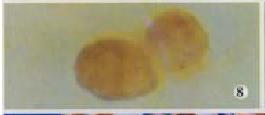
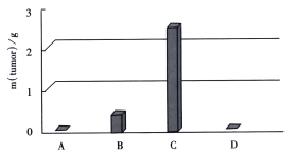
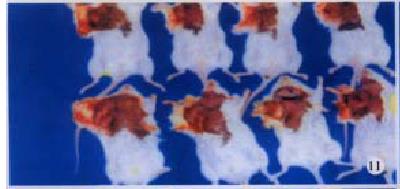
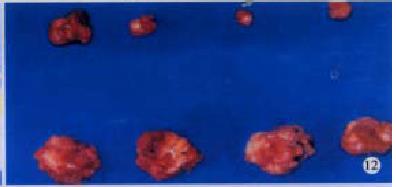
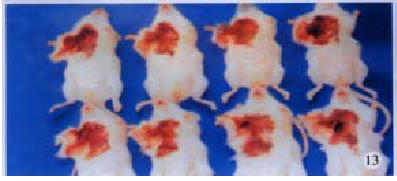
H22-DCs have some characteristics of both of their parental cells such as suspended growth, oval, flat and irregular in shape, they also have irregular shape nucleus and rich mitochondrias (Figure 7). Proliferation of H22-DC in incubation without rmGM-CSF and rmIL-4 showed slow growth and low activity. However, H22-DC incubation with rmGM-CSF and rmIL-4 was able to divide and proliferate, but compared with H22, its activity of division and proliferation was significantly decreased and their growth curve was flatter. After subcutaneous implantation over 60 d, no tumorigenesis was induced in H22-DC of mice, but induced tumorigenesis (100%) was observed in control subgroups (H22+DC and H22 subgroup) (Figure 8). The tumor weight of H22+DC subgroup mice implanted on day 14 was significantly different from that of H22 subgroup (P < 0.01, Figure 9, Figure 10, Figure 11, Figure 12).
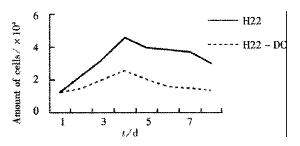
MTT assays showed that CTL activity of spleen in H22-DC group was significantly higher than that in H22+DC, H22 or PBS group (P < 0.01, Figure 13, Figure 14).
Steinman and Cohn first isolated DC from the spleen of mice in 1973[20]. Since then, scholars have successfully isolated DC from thymus, aggregated lymphoid follicle, tracheas of mice, livers of rats and human peripheral blood. In recent years, mature DC was considered able to effectively present tumor-peptide epitopes and induce cytotoxic T lymphocytes (CTL) to produce strong specific antitumor immune response[29-32]. Wu and Kufe et al[20-24] prepared DC vaccine using activated B cells and DC fused with tumor cells by traditional fusion METHODS in 1994 and 1997, respectively. This experiment was based on the established METHODS of isolation and generation of DC by chemical fusion with PEG and techniques of immunomagnetic beads, it not only apparently simplifies the complicated sorting process of traditional fusion methods, but also effectively increase the purity of cell sorting. It is simple and feasible. The CD11c monoclonal antibody N418 is specific for the integrin αx subunit of αxb2 which was the leukocytic integrin expressed on mouse splenic DC[33,34]. The principle of MACS CD11c+ cell sorting is that the cells are labelled by MicroBeads coupled with CD11c antibodies and passed through a sorting column which is placed in the magnetic field of a MACS sorter. The magnetically labelled CD11c+ DC are retained in the column while the unlabeled CD11c- cells passed away. After getting the column from the magnetic field, the magnetically retained CD11c+ DC can be eluted as the fraction of positively sorting cells. The effect of MACS sorter is confirmed by fluoroimmunoassay, PCR, FISH and FACS. The advantages of MACS are: it can process numerous cells, its sorting purity is very high, and it can be operated easily.
Much data in recent years show that DC played a very important role in the tumor immune response[35-39], especially with the develpment of gene therapy against tumor specific antigen. But at present, T cells epitopes of tumor specific antigens in most of the human cancers besides melanoma, breast cancer and ovarian cancer are not very clear[40]. Thus, cancer vaccine directly fused DC with tumor cells has become an important way in active immunotherapy of tumors[41-45]. It is simple and reliable and of practical value. At the same time, a tumor immunotherapy approach of specificly distinguishing and killing tumor cells but normal cells of host in vivo has developed[45-50].
By sorting with Mini MACS marked with mouse CD11c MicroBeads, H22-DCs have some characteristics of two parental cells, being irregular in shape. Apoptosis occurred in DC-DC and DC mixed with H22-DC respectively after 7-10 d and 10-14 d in the medium with no rmGM-CSF and rmIL-4, but H22-DCs were still alive. H22-DC could divide and proliferate quickly in the initial stage but soon their growth slowed down and their activity of dividing and proliferating reduced. We failed to establish the cell line in vitro, possibly due to the growth nature of the parental cells in vitro and loss of chromosome with the time of incubation.
After subcutaneous implantation over 60 d, H22 -DC showed no induced tumorigenesis in BALB/C mice, but did it in H22 control group (100%). It suggested that H22-DC has lost its tumorigenecity in vivo. The tumor weight in H22+DC control group was significantly different from that of H22 control group when it had been implanted for 14 d (P < 0.01). It shows that DC simply mixed with tumor cells could obviously inhibit the development of tumor in the early stage, but could not prevent the generation of tumor, which means that DC played a positive role in the course of presenting tumor antigen and inducing sepecific antitumor immune response in the early stage of tumorigenicity. By selecting the spleen of mice in H22-DC, H22+DC and H22 group on d 10 after implantation spleen CTL activity in vitro was induced in our experiment, and the results showed that the spleen CTL activity of H22-DC inoculated group was significantly higher than H22 inoculated group (P < 0.01), which suggests that the active immunity of cancer vaccine can produce specific antitumor immune protection in mice. DC and H22-DC could induce specific antitumor immune response and stimulate production of effective T lymphocytes in mice, and H22-DC induced no tumorigenesis. It indicates that DC directly fused with hepatocarcinomar cells is likely to become a helpful appreach in immunotherapy for hepatocarcinoma.
Edited by Ma JY
| 1. | Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1396] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 2. | Steinman RM, Inaba K. Myeloid dendritic cells. J Leukoc Biol. 1999;66:205-208. [PubMed] |
| 3. | Bottomly K. T cells and dendritic cells get intimate. Science. 1999;283:1124-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Cao X, Zhang W, Wang J, Zhang M, Huang X, Hamada H, Chen W. Therapy of established tumour with a hybrid cellular vaccine generated by using granulocyte-macrophage colony-stimulating factor genetically modified dendritic cells. Immunology. 1999;97:616-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999;190:125-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56-58. [PubMed] |
| 7. | Rosenzwajg M, Camus S, Guigon M, Gluckman JC. The influence of interleukin (IL)-4, IL-13, and Flt3 ligand on human dendritic cell differentiation from cord blood CD34+ progenitor cells. Exp Hematol. 1998;26:63-72. [PubMed] |
| 8. | Gilboa E, Nair SK, Lyerly HK. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol Immunother. 1998;46:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Zhang JK, Chen HB, Sun JL, Zhou YQ. Effect of dendritic cells on LPAK cells induced at different times in killing hepatoma cells. Shijie Huaren Xiaohua Zazhi. 1999;7:673-675. |
| 10. | Sun JL, Zhang JK, Chen HB, Cheng JD, Qiu YQ. Promoting eff ects of dendritic cells on LPAK cells killing human hepatoma cells. Zhongguo Zhongliu Linchuang Yu Kangfu. 1998;5:16-18. |
| 11. | Chen HB, Zhang JK, Huang ZL, Sun JL, Zhou YQ. Effects of cytokines on dendritic cells against human hepatoma cell line. Shijie Huaren Xiaohua Zazhi. 1999;7:191-193. |
| 12. | Wong C, Morse M, Nair SK. Induction of primary, human antigen-specific cytotoxic T lymphocytes in vitro using dendritic cells pulsed with peptides. J Immunother. 1998;21:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10719] [Article Influence: 397.0] [Reference Citation Analysis (0)] |
| 14. | Austyn JM. Dendritic cells. Curr Opin Hematol. 1998;5:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Liu SC, Yuan SZ. Relationship between infiltration of dendritic cells, pericancerous lymphocytic reaction and prognosis in colorectal carclinomas. Xin Xiaohuabingxue Zazhi. 1997;5:156-157. |
| 16. | Hu JY, Wang S, Zhu JG, Zhou GH, Sun QB. Expression of B7 costimulatio nmolecules by colorectal cancer cells reducestumorigenicity and induces anti-tumor immunity. World J Gastroenterol. 1999;5:147-151. [PubMed] |
| 17. | De Veerman M, Heirman C, Van Meirvenne S, Devos S, Corthals J, Moser M, Thielemans K. Retrovirally transduced bone marrow-derived dendritic cells require CD4+ T cell help to elicit protective and therapeutic antitumor immunity. J Immunol. 1999;162:144-151. [PubMed] |
| 18. | Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947-1953. [PubMed] |
| 19. | Shimizu Y, Guidotti LG, Fowler P, Chisari FV. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998;161:4520-4529. [PubMed] |
| 20. | Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 347] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Talmor M, Mirza A, Turley S, Mellman I, Hoffman LA, Steinman RM. Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur J Immunol. 1998;28:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol. 1999;31:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10719] [Article Influence: 397.0] [Reference Citation Analysis (0)] |
| 24. | Sonderbye L, Feng S, Yacoubian S, Buehler H, Ahsan N, Mulligan R, Langhoff E. In vivo and in vitro modulation of immune stimulatory capacity of primary dendritic cells by adenovirus-mediated gene transduction. Exp Clin Immunogenet. 1998;15:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Ji Y, Ling MY, Li Y, Xie H. Effect of cell fusion on metastatic ability of mouse hepatocarcinoma cell lines. World J Gastroenterol. 1999;5:22-24. [PubMed] |
| 26. | Zhang J, Liu YF, Yang SJ, Sun ZW, Qiao Q, Zhang SZ. Construction and expression of mouse/humanized scFv and their fusion to humanized mutant TNFα against hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:616-620. |
| 27. | Tanimoto K, Akbar SM, Michitaka K, Onji M. Immunohistochemical localization of antigen presenting cells in liver from patients with primary biliary cirrhosis; highly restricted distribution of CD83-positive activated dendritic cells. Pathol Res Pract. 1999;195:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Lu LG, Zeng MD, Li JQ, Hua J, Fan JG, Fan ZP, Qiu DK. Effect of lipid on proliferation and activation of rat hepatic stellate cells (I). World J Gastroenterol. 1998;4:497-499. [PubMed] |
| 29. | Li MS, Yuan AL, Zhang WD, Liu SD, Lu AM, Zhou DY. Dendritic cells in vitro induce efficient and special anti-tumor immune response. Shijie Huaren Xiaohua Zazhi. 1999;7:161-163. |
| 30. | Li MS, Yuan AL, Zhang WD, Chen XQ, Tian XH, Piao YJ. Immune response induced by dendritic cells induce apoptosis and inhibit proliferation of tumor cells. Shijie Huaren Xiaohua Zazhi. 2000;8:56-58. |
| 31. | Xiao LF, Luo LQ, Zou Y, Huang SL. Study of the phenotype of PBLs activ ated by CD28/CD80 and CD2/CD58 and acting with hepatoma cells and the restricted usage of TCR Vβ gene subfamily. Shijie Huaren Xiaohua Zazhi. 1999;7:1044-1046. |
| 32. | Zhai SH, Liu JB, Zhu P, Wang YH. CD54, CD80, CD86 and HLA-ABC express ions in liver cirrhosis and hepatocarcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:292-295. |
| 33. | Marriott I, Inscho EW, Bost KL. Extracellular uridine nucleotides initiate cytokine production by murine dendritic cells. Cell Immunol. 1999;195:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Nunez R, Grob P, Baumann S, Zuniga A, Ackermann M, Suter M. Immortalized cell lines derived from mice lacking both type I and type II IFN receptors unify some functions of immature and mature dendritic cells. Immunol Cell Biol. 1999;77:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Akbar SM, Kajino K, Tanimoto K, Yamamura Ki, Onji M, Hino O. Unique features of dendritic cells in IFN-gamma transgenic mice: relevance to cancer development and therapeutic implications. Biochem Biophys Res Commun. 1999;259:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Yamamoto K, Akbar SM, Masumoto T, Onji M. Increased nitric oxide (NO) production by antigen-presenting dendritic cells is responsible for low allogeneic mixed leucocyte reaction (MLR) in primary biliary cirrhosis (PBC). Clin Exp Immunol. 1998;114:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584-5591. [PubMed] |
| 38. | Höltl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol. 1999;161:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Ribas A, Butterfield LH, McBride WH, Dissette VB, Koh A, Vollmer CM, Hu B, Chen AY, Glaspy JA, Economou JS. Characterization of antitumor immunization to a defined melanoma antigen using genetically engineered murine dendritic cells. Cancer Gene Ther. 1999;6:523-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 347] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 41. | Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1514] [Cited by in RCA: 1698] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 42. | Chow YH, Chiang BL, Lee YL, Chi WK, Lin WC, Chen YT, Tao MH. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320-1329. [PubMed] |
| 43. | Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161:5516-5524. [PubMed] |
| 44. | Lespagnard L, Mettens P, Verheyden AM, Tasiaux N, Thielemans K, van Meirvenne S, Geldhof A, De Baetselier P, Urbain J, Leo O. Dendritic cells fused with mastocytoma cells elicit therapeutic antitumor immunity. Int J Cancer. 1998;76:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 45. | Du DW, Zhou YX, Feng ZH, Yao ZQ, Li GY. Immune responses to interleukin 12 and hepatitis B gene vaccine in H-2 d mice. Shijie Huaren Xiaohua Zazhi. 2000;8:128-130. |
| 46. | Wu MC. Progvesses in surgical treatment of primary hepatocellular carc inoma. Huaren Xiaohua Zazhi. 1998;6:921-923. |
| 47. | Zou QY, Li RB, Zheng PL, Yang LP, Chen YZ, Kong XP. Effect of embryo hepatic extracts on proliferation and differentiation of hepatoma BEL-7402 cells. Shijie Huaren Xiaohua Zazhi. 1999;7:243-245. |
| 48. | Akbar SK, Horiike N, Onji M. Prognostic importance of antigen-presenting dendritic cells during vaccine therapy in a murine hepatitis B virus carrier. Immunology. 1999;96:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Li MS, Yuan AL, Zhang WD, Liu SD, Lu AM, Zhou DY. Dendritic cells in vitro induce efficient and special anti-tumor immune response. Shijie Huaren Xiaohua Zazhi. 1999;7:161-163. |
| 50. | Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 347] [Article Influence: 13.3] [Reference Citation Analysis (0)] |