Published online Oct 15, 2001. doi: 10.3748/wjg.v7.i5.652
Revised: April 6, 2001
Accepted: April 26, 2001
Published online: October 15, 2001
AIM: To observe the gene expression change of eNOSmRNA and iNOSmRNA in the small and large intestines with acute liver failure (ALF), and to reveal the biological function of NO on the pathogenesis of ALF and multiple organs dysfunction at the molecular level.
METHODS: Sixty male Wistar rats were selected, weighing from 250 g to 350 g, and divided into 5 groups randomly: SO, ALF (6 h, 12 h), L-Arg, L-NAME, L-Arg and L-NAME, each group with 10 rats. The dose of L-Arg was 300 mg•kg¯¹, and L-NAME was 30 mg•kg¯¹, the reagents diluted by normal saline were injected through tail vein 30 minutes pre- and post-operation. The rats in the ALF group were respectively sacrificed postoperatively at 6 h, 12 h, and the rats in the other groups were sacrificed postoperatively at 6 h. The tissues of small and large intestines were harvested in 4% paraforaldehyde containing the reagent of DEPC and fixed at 6 h, embedded in paraffin, and 4 μm section was cut. The expression of eNOSmRNA and iNOSmRNA in these tissues was determined with in situ hybridization, and analyzed with the imaging analysis system of CMM-3 and SPSS statistical software.
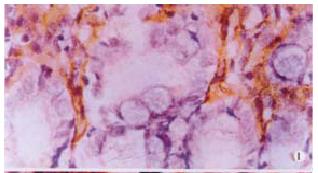
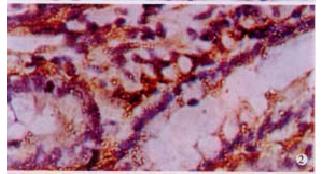
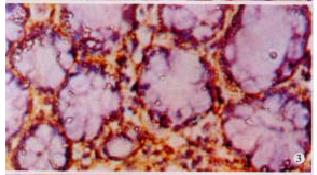
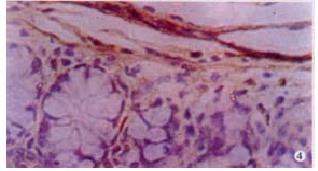
RESULTS: The expression of eNOSmRNA in the large intestine and iNOSmRNA in the small and large intestines increased significantly at 6 h after ALF, but the expression of iNOSmRNA in the small and large intestines reduced notably at 12 h after ALF (P < 0.05); the expression of eNOSmRNA in the large intestine and iNOSmRNA in the small and large intestines decreased significantly with the reagents of L-Arg at 6 h ALF, but the expression of eNOSmRNA and iNOSmRNA in the small and large intestines decreased totally with the reagents of L-NAME or association with L-Arg 6 h ALF.
CONCLUSION: The expression of eNOSmRNA in the large intestine increased notably at the early stage of ALF, NO induced by the enzyme of eNOS from the transplantation of eNOSmRNA can protect the function of the large intestine, the high expression of iNOSmRNA is involved in the damaged function of the small and large intestines. NO precursor can reduce the expression of iNOSmRNA in the small and large intestines and the damage to intestines; NOS inhibitor or association with NO precursor can totally lower the expression of eNOSmRNA and iNOSmRNA in the small and large intestines, it cannot notably influence the NOS inhibitor in the gene expression of eNOSmRNA and iNOSmRNA to supply the additional NO precursor.
- Citation: Qin JM, Zhang YD. Intestinal expressions of eNOSmRNA and iNOSmRNA in rats with acute liver failure. World J Gastroenterol 2001; 7(5): 652-656
- URL: https://www.wjgnet.com/1007-9327/full/v7/i5/652.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i5.652
Acute liver failure (ALF) can severely influence the metabolism, secretion, synthesis and biological transformation, resulting in multiple organs dysfunction (MOD). The effect of gastrointestinal ischemia on the whole body is extensive and profound, which not only causes the increase of intestinal permeability and the movement of bacteria and toxin in the intestinal cavity, but also release a large quantity of inflammatory media; neuroendocrine element after ALF is closely related to intestinal damage. Nitric oxide (NO) is a gaseous molecule with multiple biological function and “two sword” characters, and nitric oxide synthase (NOS) is a key enzyme that can synthesize NO and there are three subtypes including endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS) and neural nitric oxide synthase (nNOS). Abundant nitric NOS is distributed in gastrointestinal tract, NO induced to produce by NOS is a nonadrenergic and noncholinergic (NANC) active media, it participates extensively in various physiological and pathological functions in the intestinal tract. eNOS is Ca2+ type and mainly distributed in endothelium, regulated and controlled by Ca2+/CaM, activated by bradykinin, histamine, PAF, P substance and blood flow shear energy, and catalyzes to produce a little amount of NO that regulates vascular dilation and protects adhesive to vascular wall from platelets and neutrophils. iNOS is a non-Ca2+ type and mainly distri buted in macrophages and endothelium, not regulated and controlled by Ca2+/CaM, activated by endotoxin as well as several cytokines (such as IFN-γ, TNF-β, IL-1β), and catalyzes continuously to produce a large amount of NO that has cytotoxic effect. NO induced to produce by iNOS down-regulates the gene expression of iNOS. The negative feedback is conducted by mainly inhibiting DNA binding activity of NF-κB, it may restrict excessive production of NO under the pathophysiological conditions. The physiological and pathological effect of NO to the body depends chiefly on the quality and strength of stimulative elements, the dosage and reactive sites[1-4]. Few reports about the effects on intestinal eNOS and iNOS after ALF are available, and the gene expression of eNOS and iNOS in the intestinal tract. The study intends to disclose the biological function of NO in ALF and MOD by establishing an ALF rat model and determining the expression of eNOSmRNA and iNOSmRNA in small and large intestines so as to provide a theoretical basis for clinical treatment in ALF.
Sixty male wistar rats were obtained from the Experimental Animal Center of Hunan Medical University, weighing from 250 g to 350 g, and divided into 5 groups randomly: sham operation, ALF (6 h, 12 h), L-Arg, L-NAME, L-Arg and L-NAME group. Each group had 10 rats.
L-arginine (L-Arg) NO precusor, 300 mg•kg¯¹, obtained from Shanghai Chemical Reagent Company.
N-nitric -L-arginine methyl ester (L-NAME) NOS inhibitor, 30 mg•kg¯¹, obtained from Sigma Chemical Company.
eNOSmRNA and iNOSmRNA in situ hybridization kit Obtained from Wuhan Boster Biological Company. Oligoneucleotide probe sequences deriving from human gene: AI925269 GI5661233--① 121---155 (probe sequence --GAGGACTTTCTCCGTTCTCCTTAGATTGTAAGCTG---); ② 661---695 (probe sequence---ACGTGCCAGACGTCCTGCAACAACCGTACCATTTA---), rat gene AJ011116 GI3676237 -- and 216---250 (probe sequence---CCTAAGACCGTTCTGGCTAATGTGCTGTAA CTCTA---).
The ALF rat model was established by 90% hepatectomy according to Lu et al′s[5] method. L-Arg or L-NAME diluted with normal saline was preoperatively and postoperatively injected into the tail vein 30 min after operation in the L-Arg group or L-NAME group; L-Arg was injected preoperatively, L-NAME was preoperatively injected at a 30 min interval, normal saline was injected 30 min preoperatively and postoperatively to SO group and ALF group. The animals were sacrificed at 6 h and 12 h in ALF group after operation, the animals of the other groups were sacrificed at 6 h after operation. The tissues of small and large intestines were fixed in 4% paraformaldehyde containing the reagent of DEPC for 6 h, dehydrated gradually, embedded in paraffin, and cut into 4 μm sections.
The detailed manipulations were conducted according to introduction of reagent kits, phosphate buffered saline was used as the negative control in stead of oligoneucleotide probe, and the tissue of breast cancer as positive control. Five views were randomly selected in each tissue sections, measured under the 10 × 20 fold statistics microscope and analyzed with the imaging analysis system of CMM-3. The mean absorbance optical density (OD) was measured, P value less than 0.05 was regarded as having significant difference.
The expressions of eNOSmRNA in large intestine and iNOSmRNA in small and large intestines in ALF increased significantly at 6 h (Figure 1 Figure 2 Figure 3), and the expressions of iNOSmRNA in small and large intestines in ALF decreased significantly at 12 h (P < 0.05). The expressions of eNOSmRNA in large intestine and iNOSmRNA in small and large intestines in ALF decreased significantly at 6 h using L-Arg, but the expressions of eNOSmRNA and iNOSmRNA in small and large intestines in ALF decreased significantly at 6 h using L-NAME or combined with L-Arg (Figure 4, P < 0.05). The expression of eNOSmRNA and iNOSmRNA in bowel tissues are compared among all groups in Table 1.
| Group | t/p | Gene expression | Small intestine | Large intestine |
| SO | 6 | eNOSmRNA | 0.05 ± 0.02 | 0.05 ± 0.02a |
| iNOSmRNA | 0.06 ± 0.02c | 0.06 ± 0.02c | ||
| ALF | 6 | eNOSmRNA | 0.10 ± 0.06e | 0.18 ± 0.06e |
| iNOSmRNA | 0.19 ± 0.05 | 0.33 ± 0.08 | ||
| ALF | 12 | eNOSmRNA | 0.05 ± 0.02 | 0.10 ± 0.04e |
| iNOSmRNA | 0.05 ± 0.01c | 0.17 ± 0.03c | ||
| L-Arg | 6 | eNOSmRNA | 0.06 ± 0.01 | 0.06 ± 0.01 |
| iNOSmRNA | 0.06 ± 0.02c | 0.07 ± 0.02c | ||
| L-NAME | 6 | eNOSmRNA | 0.06 ± 0.01 | 0.09 ± 0.01 |
| iNOSmRNA | 0.07 ± 0.02c | 0.16 ± 0.03c | ||
| L-Arg+L-NAME | 6 | eNOSmRNA | 0.06 ± 0.01 | 0.07 ± 0.02 |
| iNOSmRNA | 0.08 ± 0.03c | 0.14 ± 0.04c |
ALF is a cooperative consequence of endotoxicemia, microcirculation dysfunction as well as inflammatory cells (such as macrophage, lymphocyte, etc) that release inflammatory mediators and cytokines (such as TNF, IFN, IL-1, etc) when stimulated, but definite mechanism remains unclear. The damage and decrease of phagocytosis in the liver result in enteral endotoxicemia and decrease in Na+/K+-ATP enzyme activity of liver cellular membrane, it causes intrahepatic cholestatic jaundice, dramatical decrease in glomerular filterable rate (GFR), increase in serum Cr, BUN and decrease in urine narium. Collection of glutamine in cerebral cells results in hepatic coma because of tyrosine, tryptophane and phenylalanine in brain, it suggests that enteral endotoxicemia is common substantial basis causing liver function failure syndrome, important pathophysiological stage of liver function failure, and mutual cause and result with liver function failure. It produces poison to hepatocytes, results in microcirculation dysfunction, aggravates liver damage and inhibits liver regener ation. Irreversible liver damage is also an initiate factor of subsequent multiple organs function failure because of liver function failure, it causes not only jaundice, haemorrhage but also renal function failure and hepaticcoma[6,7]. Deficient L-arginine inhibits intrahepatic L-Arg/NO pathway and influences systematic blood flow when liver ischemia-reperfusion, L-arginine injected by portal vein inhibits arginase release in liver, reverses L-Arg/NO pathway, and mitigates liver injury[8,9]. Administration of arginine into intestinal tract can mitigate liver function impairment by acute liver injury, activate immune system of intestinal mucosa and regulate and decrease bacterial translocation[10]. GSH can participate in the regulation of iNOS activity, up-regulate the expression of iNOSmRNA, aggravate liver injury under stress condition[11]. Deficient TNF-α and IL-6 caused hepatic steatosis and high mortality, replenishment of TNF-α and IL-6 restored the hepatocyte regeneration[12]. NO can prevent cell apoptosis induced by TNF, and activate extra endothelial regulating kinase 1 and 2, inhibit NF-κB activation in cell[2,13-16]. Furthermore, NO inhibits Caspase-3-like proteinase and cell apoptosis, iNOS gene transfection mediated by adenovirus into hepatocytes can produce NO, which efficiently inhibits Caspase- 3-like proteinase activity, and local apoptosis in hepatocyte[17-20]. IL-1β can induce iNOS expression in hepatocyte by activating iNOS primer by IFN-γ, and produce a large amount of extra cellular matrix (ECM) such as hyaluronan, which induced iNOS to produce NO and form ONOO-, and aggravate tissue injury by NF-κB signal transduction when liver is damaged, moreover, stabilize microcirculation and protect against liver injury. The effect of protection or impairment on the liver mainly depends on the NO concentration and cell derivation[21-23]. NO induced by iNOS in endotoxemia is closely related to liver function impairment, but NO induced early by hepatocyte cNOS can protect liver tissue[24,25]. The dual stimulation of endotoxemia and ischemia-reperfusion influenced microvascular blood flow and impaired liver tissue by selectively inhibiting eNOS or/and iNOS[26,27]. Enterogenous endotoxin permeated blood circulation and stimulated the expression of iNOS in intestinal tissues by portal-cavity lateral circulation. NO participates in hyperdynamic circulation of portal hypertension and general hyperdynamic circulation in cirrhosis[28,29]. Selectively inhibiting eNOS can cause vascular constriction and thrombosis and lower liver perfusion pressure, but had no influence on portal pressure; the difference may be related to different time interval of endotoxin and NOS inhibitor[30]. The expression of NOSmRNA decreased remarkably in the kidney of endotoxemia of cirrhosis, deficient NO or low sensitivity to NO in renal vessels can aggravate renal damage, and promote hepatorenal syndrome (HRS)[31]. Endogenous NO has anti-inflammatory function and inhibits neutrophiles to adhere to vascular endothelial cells and translocate from vessels. NO deriving from aminoformylp enicillamine can alleviate small intestine impairment induced by endotoxin and plasma leakage, suggesting that endogenous NO can maintain intestinal mucosal microvascular integrity, dilate intestinal vessel and improve gut microvasculation, react with superoxide (O2-) to be antioxidation, contribute to recovery of impaired enteric function. L-arginine and solution nitric prusside caused absorption of water and ions, but NOS inhibitor (L-NAMA) caused secretion of water and ions in the ileum, synchronous infusion of L-arginine and L-NAME cause absorption of water and ions in the ileum, indicating that endogenous NO can mediate absorption of water and ions in the ileum, maintain homeostasis of water and electrolytes. Neutrophil activation in circulation has been implicated in the capillary leakage during ischemia-reperfusion. It suggests that endogenous NO can maintain the capillary integrity and prevent macromolecular leakage. Capillary permeability and macromolecular leakage are critical factors causing ARDS during intestinal ischemia-reperfusion, and increased endogenous NO can maintain normal capillary function. It is very important to prevent systemic response such as ARDS caused by intestinal ischemia-reperfusion[32-36]. Endotoxemia results in increased constitutive NOS and inducible NOS enzyme activities in the jejunum and ilem. LPS can upregulate constitutive NOS and inducible NOS in jejunal and ileal smooth muscle oxyhemoglobin increased intestinal transit in nonendotoxemia. NO may play a major role in mediating the rapid intestinal transit induced by endotoxemia. Hemoglobin attenuated the effect of endotoxin on intestinal motor function. Synthetic hemoglobins currently being a test as blood substitutes may be of therapeutic value in treating intestinal dysfunction complicated with sepsis [37-41]. However, the gene expression increase of iNOAmRNA in the intestinal tract produced a large amount of NO, slowed intestinal transit, promoted intestinal bacteria overgrowth during endotoxemia, and reacted with superoide (O2-) to form peroxynitrite anion (ONOO-), causing inactivation of mitochondrial aconitase in entercytes and activation of poly-adenosine diphosphate ribosyl polymerase, leads to depletion of intracellular ATP stores by interfering with mitochondrial respiration and intestinal mucosal barrier dysfunction, prolonged the exposure of cells to large amounts of NO may inhibit cell respiration, cause maldistribution of regional blood flow and cell damage, increase gut permeability; but inhibition of cNOS can decrease dramatically the blood flow of intestine, increase the damage of enteric hemorrhage by induction of LPS, even small dosage of LPS could cause enteric hemorrhage. LPS can inhibit cNOS, decreasing NO and increasing active oxidizing free radicals. L-Arg can protected the enteric hemorrhage from LPS or LPS associated with L-NAME. Inhibiting NO release can increase active oxygen production, and contribute to acute enteric damage by LPS[42-46]. Mishima et al[47] found that the iNOS-deficient mice could resist enterocyte apoptosis induced by LPS, and attenuate intestinal mucosal injury. Bacterial translocation induced by LPS was not found, suggesting that LPS could induce iNOS activation and increase NO production, resulting in intestinal mucosal injury and bacterial translocation, inhibiting iNOS can decrease NO production and prevent intestinal mucosal injury and bacterial translocation. Fruchterman et al[1,48-50] observed that decrease of activity of cNOS and NO production in the small intestinal endothelial cell resulted in intestinal microvascular dysfuncti on such as low perfusion, platlets and neutrophils converged into postcapillary venule when hemorrhage shock and resuscitation occurred. ONOO- deriving from NO connection with O2- could decrease NO production, inhibit the synthesis of prostaglandin I2 (PGI2), impaie endothelial cell mitochondrial and lead to endothelial cell dysfunction and intestinal nucosal impairment. NOSmRNA expression in colonic mucosa increased remarkably, especially iNOSmRNA, suggesting that vasodilation and blood flow increase because of increased NO production in colonic tissues, mucosal congestion was active not passive, inhibtion of iNOS and NO production may protect or mitigate the pathogenesis of portal hypertensive enteropathy (PHE). NO plays an important role in maintaining gastrointestinal vascular integrity and gastrointestinal motility under physiological and pathophysiological circumstances, it can relax vascular and gastrointestinal tract smooth muscle and contributes to LPS-induced intussusception pathogenesis by activating soluble guanylate cyclase (sGC) to increase intracellular cGMP, inhibiting NOS worsens the intestinal injury induced by ischemia-reperfusion and endotoxin, decreases the incidence of intussusception, but L-Arg can increase the incidence of intussusception, suggesting that endogenous NO plays an important in regulating normal and pathop hysiological circumstance by activating sGC and altering intracellular level of cGMP[51,52]. Our study indicated that the expression of eNOSmRNA increased dramatically at early stage of ALF, NO produced by eNOS can keep the integrity of enteral microvascular mucosa, expand blood vessel in enteral mucosa, improve microcirculation, and has antioxidant function by connecting with superoxide anion and protects enteral function from damage. but the high expression of iNOSmRNA in large intestine can induce iNOS synthesis constantly and produce a large amount of NO, slow enteric peristalsis, promote bacterial outgrowth, increase calciumion concentration, result in protective barrier dysfunction in enteral epithelium, penetration and bacterial translocation increase. The low expression of eNOSmRNA in small and large intestines might be related to consumable increase of eNOS, furthermore, the low expression of iNOSmRNA in small and large intestines might be related to producing excessive NO locally can and feedbackly inhibiting the expression of iNOSmRNA after replenishing exogenous larginine. L-Arg contributes to water and electrolyte absorption in small intestine and plays an important role in keeping microcirculatory stability, suggesting that replenishing exogenous NO precursor can reduce the enteral expression of iNOSmRNA and beneficial to organism, NOS inhibitor can significantly influence the gene expression of both enzymes in large intestine, especially reducing the eNOSmRNA expression more significantly. NO produced by high expression of iNOSmRNA may influence local microcirculation and integrity in enteral mucosa, and contribute to enteral bacterial translocation in ALF. It is similar to influence to eNOSmRNA and iNOSmRNA expression using NO precusor connecting with NOS inhibitor or only using NOS inhibitor, and indicating that replenishing exogenous NO precusor can not significantly influence the effect of NOS inhibitor on both gene expression of eNOS and iNOS. It is necessary to further on the mechanism of mutual interaction with them.
Edited by Ma JY
| 1. | Wang QG, He LY, Chen YW, Hu SL. Enzymohistochemical study on burn effect on rat intestinal NOS. World J Gastroenterol. 2000;6:421-423. [PubMed] |
| 2. | Taylor BS, Kim YM, Wang Q, Shapiro RA, Billiar TR, Geller DA. Nitric oxide down-regulates hepatocyte-inducible nitric oxide synthase gene expression. Arch Surg. 1997;132:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Rachmilewitz D. Role of nitric oxide in gastrointestinal tract. World J Gastroenterol. 1998;4:28-29. |
| 4. | Peng X, Wang SL. Nitric oxide and gastroenteric movement. Huaren Xiaohua Zazhi. 1998;6:445-446. |
| 5. | Lu Y, Pan C, Liu X, Meng L, Qin Z, Zhang M. [The treatment of rat fulminant hepatic failure by auxiliary partial heterotopic liver transplantation: experimental study]. Zhonghua Wai Ke Za Zhi. 1998;36:519-521. [PubMed] |
| 6. | Han DW. Enteral endotoxicemia and liver failure. Linchuang Gandan Zazhi. 1996;1:50-53. |
| 7. | Wu CT, Huang XC, Li ZL. Increased intestinal permeability and intestinal bacterial transposition. Shijie Huaren Xiaohua Zazhi. 1999;7:605-606. |
| 8. | Shiraishi M, Hiroyasu S, Nagahama M, Miyaguni T, Higa T, Tomori H, Okuhama Y, Kusano T, Muto Y. Role of exogenous L-arginine in hepatic ischemia-reperfusion injury. J Surg Res. 1997;69:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Chen XH, Li ZZ, Bao MS, Zheng HX. Effect of nitric oxide on liver ischemia/reperfusion injury in rats in vivo. Shijie Huaren Xiaohua Zazhi. 1999;7:295-297. |
| 10. | Adawi D, Kasravi FB, Molin G, Jeppsson B. Effect of Lactobacillus supplementation with and without arginine on liver damage and bacterial translocation in an acute liver injury model in the rat. Hepatology. 1997;25:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Harbrecht BG, Di Silvio M, Chough V, Kim YM, Simmons RL, Billiar TR. Glutathione regulates nitric oxide synthase in cultured hepatocytes. Ann Surg. 1997;225:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 755] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 13. | Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997;272:31138-31148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 673] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 14. | Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;240:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 387] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 15. | Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220-4226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 324] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Togashi H, Sasaki M, Frohman E, Taira E, Ratan RR, Dawson TM, Dawson VL. Neuronal (type I) nitric oxide synthase regulates nuclear factor kappaB activity and immunologic (type II) nitric oxide synthase expression. Proc Natl Acad Sci USA. 1997;94:2676-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Dimmeler S, Haendeler J, Nehls M, Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med. 1997;185:601-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 671] [Cited by in RCA: 654] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 18. | Mannick JB, Miao XQ, Stamler JS. Nitric oxide inhibits Fas-induced apoptosis. J Biol Chem. 1997;272:24125-24128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 222] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Ou J, Carlos TM, Watkins SC, Saavedra JE, Keefer LK, Kim YM, Harbrecht BG, Billiar TR. Differential effects of nonselective nitric oxide synthase (NOS) and selective inducible NOS inhibition on hepatic necrosis, apoptosis, ICAM-1 expression, and neutrophil accumulation during endotoxemia. Nitric Oxide. 1997;1:404-416. [PubMed] |
| 20. | Tzeng E, Billiar TR, Williams DL, Li J, Lizonova A, Kovesdi I, Kim YM. Adenovirus-mediated inducible nitric oxide synthase gene transfer inhibits hepatocyte apoptosis. Surgery. 1998;124:278-283. [PubMed] |
| 21. | Wang Z, Wang M, Carr BI. The inhibitory effect of interleukin 1beta on rat hepatocyte DNA synthesis is mediated by nitric oxide. Hepatology. 1998;28:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Schroeder RA, Gu JS, Kuo PC. Interleukin 1beta-stimulated production of nitric oxide in rat hepatocytes is mediated through endogenous synthesis of interferon gamma. Hepatology. 1998;27:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Rockey DC, Chung JJ, McKee CM, Noble PW. Stimulation of inducible nitric oxide synthase in rat liver by hyaluronan fragments. Hepatology. 1998;27:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Smith RE, Robinson NM, McPeake JR, Baylis SA, Charles IG, Heaton ND, Moncada S, Williams R, Martin JF. Induction and role of NO synthase in hypotensive hepatic failure. Arterioscler Thromb Vasc Biol. 1997;17:3079-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Norby SW, Weyhenmeyer JA, Clarkson RB. Stimulation and inhibition of nitric oxide production in macrophages and neural cells as observed by spin trapping. Free Radic Biol Med. 1997;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Lawson JA, Jaeschke H. Differential effect of 2-aminoethyl-isothiourea, an inhibitor of the inducible nitric oxide synthase, on microvascular blood flow and organ injury in models of hepatic ischemia-reperfusion and endotoxemia. Shock. 1998;10:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Strand OA, Kirkebøen KA, Giercksky KE. Inhibition of nitric oxide synthase does not affect survival in a rat model of abdominal sepsis. Scand J Clin Lab Invest. 1997;57:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci USA. 1998;95:13829-13834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 192] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Mizumoto M, Arii S, Furutani M, Nakamura T, Ishigami S, Monden K, Ishiguro S, Fujita S, Imamura M. NO as an indicator of portal hemodynamics and the role of iNOS in increased NO production in CCl4-induced liver cirrhosis. J Surg Res. 1997;70:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Vos TA, Gouw AS, Klok PA, Havinga R, van Goor H, Huitema S, Roelofsen H, Kuipers F, Jansen PL, Moshage H. Differential effects of nitric oxide synthase inhibitors on endotoxin-induced liver damage in rats. Gastroenterology. 1997;113:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Battista S, Bar F, Mengozzi G, Zanon E, Grosso M, Molino G. Hyperdynamic circulation in patients with cirrhosis: direct measurement of nitric oxide levels in hepatic and portal veins. J Hepatol. 1997;26:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Degnim AC, Morrow SE, Ku J, Zar HA, Nakayama DK. Nitric oxide inhibits peroxide-mediated endothelial toxicity. J Surg Res. 1998;75:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Ou J, Carlos TM, Watkins SC, Saavedra JE, Keefer LK, Kim YM, Harbrecht BG, Billiar TR. Differential effects of nonselective nitric oxide synthase (NOS) and selective inducible NOS inhibition on hepatic necrosis, apoptosis, ICAM-1 expression, and neutrophil accumulation during endotoxemia. Nitric Oxide. 1997;1:404-416. [PubMed] |
| 34. | Murr MM, Balsiger BM, Farrugia G, Sarr MG. Role of nitric oxide, vasoactive intestinal polypeptide, and ATP in inhibitory neurotransmission in human jejunum. J Surg Res. 1999;84:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Riordan SM, McIver CJ, Thomas DH, Duncombe VM, Bolin TD, Thomas MC. Luminal bacteria and small-intestinal permeability. Scand J Gastroenterol. 1997;32:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Riordan SM, McIver CJ, Williams R. Liver damage in human small intestinal bacterial overgrowth. Am J Gastroenterol. 1998;93:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Cullen JJ, Mercer D, Hinkhouse M, Ephgrave KS, Conklin JL. Effects of endotoxin on regulation of intestinal smooth muscle nitric oxide synthase and intestinal transit. Surgery. 1999;125:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Cullen JJ, Caropreso DK, Ephgrave KS, Hemann LL, Hinkhouse MM. The effect of endotoxin on canine jejunal motility and transit. J Surg Res. 1997;67:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Cullen JJ, Doty RC, Ephgrave KS, Hinkhouse MM, Broadhurst K. Changes in intestinal transit and absorption during endotoxemia are dose dependent. J Surg Res. 1999;81:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Gould SA, Moore EE, Hoyt DB, Burch JM, Haenel JB, Garcia J, DeWoskin R, Moss GS. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg. 1998;187:113-20; discussion 120-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 221] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Hellström PM, al-Saffar A, Ljung T, Theodorsson E. Endotoxin actions on myoelectric activity, transit, and neuropeptides in the gut. Role of nitric oxide. Dig Dis Sci. 1997;42:1640-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Menconi MJ, Unno N, Smith M, Aguirre DE, Fink MP. Nitric oxide donor-induced hyperpermeability of cultured intestinal epithelial monolayers: role of superoxide radical, hydroxyl radical, and peroxynitrite. Biochim Biophys Acta. 1998;1425:189-203. [PubMed] |
| 43. | Arkovitz MS, Wispé JR, Garcia VF, Szabó C. Selective inhibition of the inducible isoform of nitric oxide synthase prevents pulmonary transvascular flux during acute endotoxemia. J Pediatr Surg. 1996;31:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294-5299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14827] [Cited by in RCA: 17531] [Article Influence: 381.1] [Reference Citation Analysis (0)] |
| 45. | Szabó C, Saunders C, O'Connor M, Salzman AL. Peroxynitrite causes energy depletion and increases permeability via activation of poly (ADP-ribose) synthetase in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 1997;16:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Fruchterman TM, Spain DA, Matheson PJ, Martin AW, Wilson MA, Harris PD, Garrison RN. Small intestinal production of nitric oxide is decreased following resuscitated hemorrhage. J Surg Res. 1998;80:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Mishima S, Xu D, Lu Q, Deitch EA. Bacterial translocation is inhibited in inducible nitric oxide synthase knockout mice after endotoxin challenge but not in a model of bacterial overgrowth. Arch Surg. 1997;132:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Kelly E, Shah NS, Morgan NN, Watkins SC, Peitzman AB, Billiar TR. Physiologic and molecular characterization of the role of nitric oxide in hemorrhagic shock: evidence that type II nitric oxide synthase does not regulate vascular decompensation. Shock. 1997;7:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Flynn WJ, Pilati D, Hoover EL. Xanthine oxidase inhibition after resuscitated hemorrhagic shock restores mesenteric blood flow without vasodilation. Shock. 1997;8:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | McGill SN, Ahmed NA, Christou NV. Increased plasma von Willebrand factor in the systemic inflammatory response syndrome is derived from generalized endothelial cell activation. Crit Care Med. 1998;26:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Wang P, Liu B, Ou HS, Tong LJ, Yang J, Tang CS. Nitric oxide synthase/ nitric oxide pathway mediates intussusception pathogenesis in rats. Chin Med J. 1999;112:1016-1019. |
| 52. | Nissan A, Zhang JM, Lin Z, Haskel Y, Freund HR, Hanani M. The contribution of inflammatory mediators and nitric oxide to lipopolysaccharide-induced intussusception in mice. J Surg Res. 1997;69:205-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |