INTRODUCTION
To date, the major therapy for rectal carcinoma is extensive abdomino-perineal resection[1]. Unfortunately, after resection of rectal carcinoma, many patients still die of blood-borne metastases, usually in the liver or lungs, or local pelvic recurrence[2,3], which is the major cause of morbidity and mortality in patients with rectal carcinoma. Pre-or postoperative radiotherapy can reduce the incidence of local recurrence[4-7], but even with moderately high radiation doses, many patients are not locally controlled and have distant metastases[8,9]. The reason for this may be low intrinsic radiotherapy in at least some of the tumors.
Fast neutrons were introduced for cancer therapy in the 1970s. It has been reported that fast neutron therapy is effective for some carcinomas[10,11]. In addition, apoptosis induced by X-rays has been reported[12,13], but apoptosis induced by fast neutron rarely reported. Therefore, in the present study fluorescent staining of DNA is used to detect not only whether irradiation with fast neutron induces apoptosis, but also whether the difference in the level of apoptosis exists between X-rays and neutron induced Chinese rectal carcinoma cell lines. Furthermore, p53 and Bcl-2 gene expression were detected by immunohistochemical methods for Chinese human rectal carcinoma cell line with various doses of X-rays and neutrons, we could thereby compare the level of p53 and Bcl-2 gene expression induced by X-rays and neutrons.
MATERIALS AND METHODS
HR8348 cells taken from poorly differentiated adenocarcinoma of the rectum were grown as a monolayer in Dulbecco's Modified Eagle Medium supplemented with 100 mL·L-1 heat-inactivated fetal bovine serum and subcultured every other day. Culture conditions were 37 °C in a humidified atmosphere buffered by 50 mL·L-1 CO2 in air and 56 g·L-1 sodium hydrogen carbonate, pH7.4. Twenty-four h after passaging, exponential cell cultures were irradiated at room temperature with a single dose of 6-Mv X-rays from Varian linear accelerator (Cancer Institute and Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College). The dose rate was about 250 cGy·min-1. The SSD (source-surface distance) was 100 cm. The PDD (percentage of depth dose) was 100%. The field of radiation was about 32.6 cm × 30.5 cm. Cells were radiated with 0, 2, 4, 6, 8, 10 Gy.
Fast-neutron-rays radiation conditions
The conditions of cells culture while cells were radiated by fast-neutron rays from electron cyclotron (High Energy Physics Institute, Chinese Academy of Sciences) were the same as by X-rays. The SSD was about was about 150 cm. The PDD was 93.68%. The field of radiation was about 18.0 cm × 18.0 cm. Cells were irradiated with 0, 0.67, 1.34, 2.01, 2.68, 3.34 Gy (the same effects of radiobiology as one-third of the equivalent dose of X-rays).
Apoptosis assay
Cells were plated in 100 mm2 tissue culture flask (106 cells/flask) for determination of apoptotic fraction. The cells were irradiated by various dosages of X-rays (0, 2, 4, 6, 8, 10 Gy respectively) and fast-neutron-rays (0, 0.67, 1.34, 2.01, 2.68, 3.34 Gy respectively) in exponential phase, approximately 24 h later. After replacement with fresh culture medium, the cell samples were exposed at room temperature to graded doses of X-rays or fast-neutron-rays. After radiation, the tissue culture flasks were incubated at 37 °C for 6, 24, 48 and 72 h respectively prior to the assessment of apoptosis. The cells (detached cells and attached cells) trypsiniszed were collected together,spread on glass slides and fixed with 900 mL·L-1 methonal and 100 mL·L-1 glacial acetic acid. Then, the slides were stained with 1 g·L-1 Hoechest 33342 in PBS for 10 min, and rinsed briefly in water. The nuclear morphology was then examined by fluorescence microscopy.
Apoptotic cells were counted microscopically, according to published criteria[14-18]. The frequency was expressed as the number of apoptotic cells per 100 cells (apoptotic index). 1500 cells per data point were counted. Additionally, apoptosis was evaluated by gel electrophresis. Cells were washed once with PBS and digested overnight at 37 °C with 100 μg/mL proteinase K in 20 mmol·L-1 TRIS-HCl (pH8.0), 10 mmol·L-1 NaCl, 1 mmol·L-1 EDTA and 10 g·L-1 SDS. DNA was extracted with phenol and chloroform, then precipitated with 1/10 volumes of 3 mol·L-1 sodium acetate and 4 volumes of ethanol, dissolved in TE buffer (pH8.0) and digested with RNAase. DNA samples were electrophoresed on a 15 g·L-1 agrose gel, stained with ethidium bromide, and visualized with an UV illuminator. Moreover, the method of TdT (terminal deoxynucleotidyl transferase)-mediated dUTP-biotin nick end labeling (TUNEL), was performed according to the instructions in situ cell death detection kit (Boehringer Mannheim, Germany).
Immunohistochemistry
The glass slides with cells were immersed in 3 mL·L-1 hydrogen peroxidase in phosphate buffered saline (PBS) for 5 min to block endogenus peroxidase activity. After washing with PBS, the slides were treated with 100 mL·L-1 normal goat serum to reduce nonspecific antibody binding. For antigen retrievals the slides were placed in an antigen retrieval solution (10 mmol·L-1 citric acid monohydrate pH6.0) and heated in a microwave oven for 10 min. After washing with PBS the slides were incubated for 1 h with anti-p53 and Bcl-2 monoclonal antibody. After further washing with PBS, slides were incubated with biotinylated rabbit anti-mouse second-stage antibody. After washing further with PBS, peroxidase-conjugated streptavidin was applied. Peroxidase activity was demonstrated by adding diaminobenzidine as a chromogen. The slides were counter-stained with hemotoxylin. Negative control slides were processed without the primary antibody. Positive control slides were processed from a nasopharyngeal squamous low-differentiated carcinoma previously shown to express high levels of p53 protein and Bcl-2 protein. Immunostained slides of both test and control slides were independently scored by two of the authors. In rare cases of disagreement, consensus was reached after discussion. For p53 protein, only nuclear staining was considered significant. For Bcl-2 protein, only cytoplasm staining was considered significant. A semiquantitative scale was used for grading of positive immunostaining, depending on the intensity of nuclear staining and the percentage of the stained nuclei.
RESULTS
Apoptosis in relation to time and dose
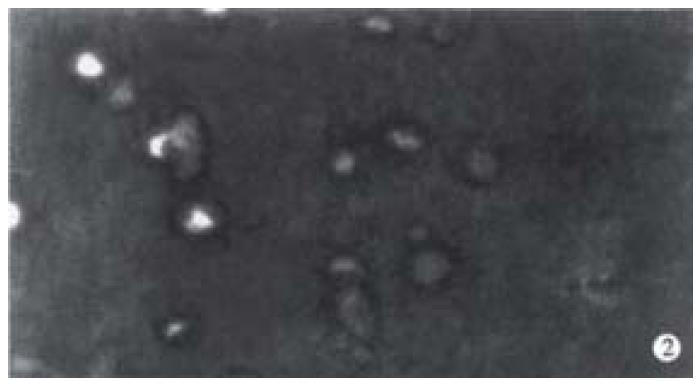
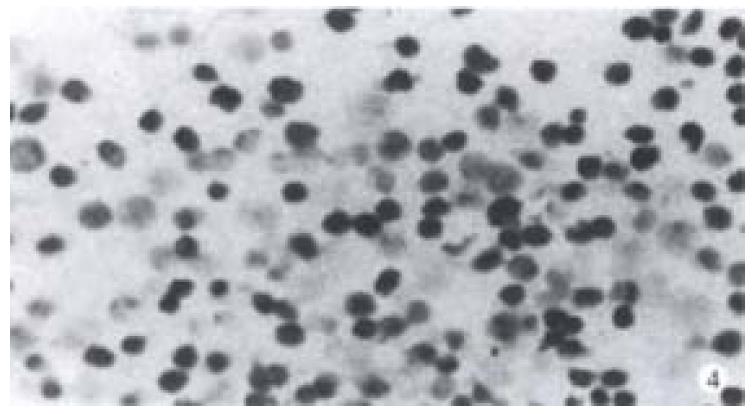
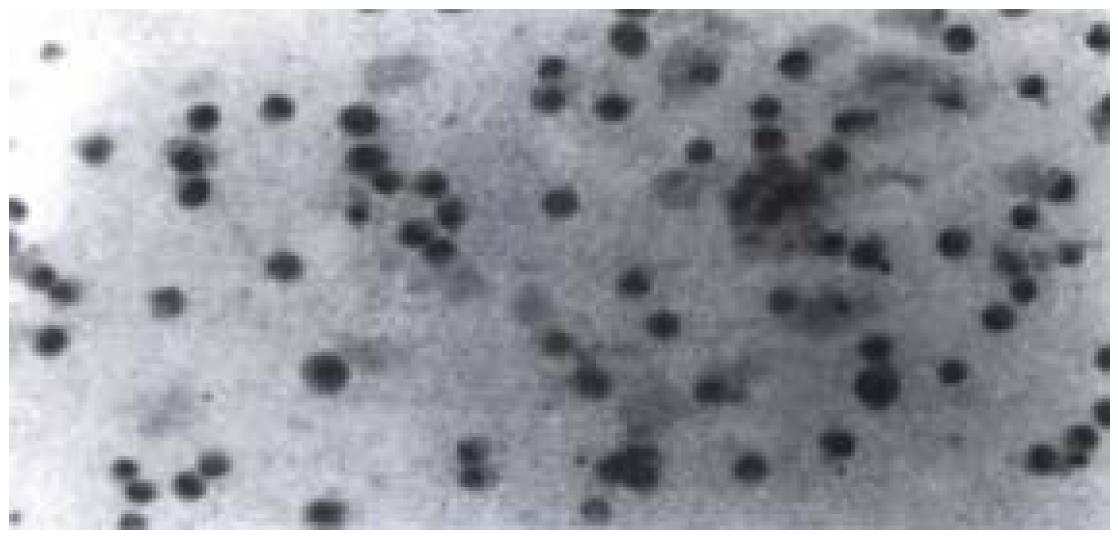
Increase of apoptotic cells above the spontaneous level (4.1%) was detected up to 2 Gy (or 0.67 Gy). Apoptotic index (percentage of apoptotic cells) increased with the increase of radiation dose and time after irradiation by X-rays and fast-neutron-rays. No cell apoptosis plateau level 72 h after radiation was observed. At the same point of time, the AI of cells induced by fast-neutron-rays was higher than AI of cells induced by X-rays. (Figure 1A, Figure 1B, Figure 1C). Figure 2 represents the features of cell shrinkage, chromatin condensation and margination of cell apoptosis induced by X-rays and fast-neutron-rays respectively in Chinese rectal carcinoma cell line. We were able to visualize the characteristic DNA ladder pattern induced by X-rays and fast-neutron-rays (Figure 3). TUNEL clearly revealed a distinct pattern of nuclear staining (Figure 4).
Figure 1 (A) AI at different time intervals after radiation of various doses of X-rays and (B) fast-neutron-rays.
AI of cells induced by X-rays and fast-neutron-rays of various doses gradually increased with the increase of dose and time. (C) Comparison of AI between X-rays and N-rays. At the same point of time, AI of cells induced by fast-neutron-rays was higher than that induced by X-rays.
Figure 2 Hoechest 33342 fluorescence staining of cells radiated by fast-neutron-rays shows the special morphological features of apoptotic cells: cell shrinkage, chromatin condensation, lunated margination.
Figure 3 Effect of X-rays on DNA fragmentation in HR 8348 cells.
Cells were incubated for 6 h after radiation by X-rays. DNA fragmentation was examined by agrose gel analysis, as described in Materials and methods. A, 885 bp and 585 bp DNA marker; B, Control; C, 2 Gy; D, 8 Gy; E, 10 Gy.
Figure 4 Positive nuclear staining of cells irradiated by 8 Gy X-rays with TUNEL was observed.
p53 and Bcl-2 immunostaining related to irradiation dose
High expression of p53 protein and Bcl-2 protein were observed in cells without radiation, and the percentage of positive cells was about 90%. After radiation with various dosages of X-rays, no apparent decrease in percentage of p53 positive cells (about 80%) was observed. However, the of p53 positive cells changed from deep yellow to light yellow. While the percentage and degree of Bcl-2 positive cells showed no apparent decrease when compared with p53 protein. As for fast-neutron-rays, it didn't lower the percentage or the degree of p53 and Bcl-2 positive cells significantly (Figure 5).
Figure 5 p53 positive nuclear staining of cells irradiated by 8 Gy X-rays with immunohistochemistry staining was seen.
Nuclei of cells appeared in light yellow.
DISCUSSION
Our results indicate that apoptosis induced by X-rays and fast-neutron-rays in Chinese rectal carcinoma cell line is time-dose dependent. Moreover, the level of apoptosis induced by fast-neutron-rays is higher than by X-rays, which suggests rectal cancer cells are more radiosensitive to fast-neutron-rays than to X-rays. Fast-neutron-rays is particulate radiation and high linear energy transfer (LET) radiation, with no charged particles. X-ray is electromagnetic radiation and low linear energy transfer radiation. These exists difference in radiosensitivity of cells between fast-neutron-rays and X-rays, because: ① anoxic cells exist in most tumor cells[19,20], and ionizing density causes the oxygen enhancement ratio (OER) of high LET radiation to be lower than that of low LET radiation, that is, the radiosensitivity of high LET radiation is less dependent on oxygen in cells than low LET radiation; ② fast neutrons are densely ionizing particles, with a high relative biological effectiveness relative to X-rays (RBE); and ③ sublethal damage repair produced by high LET radiation is lower than that by low LET radiation. So far, the molecular basis of their properties is not yet entirely understood. The term apoptosis was first used by Kerr, Wyllie and co-workers to describe a particular form of cell death, different from necrosis[21-23]. Much evidence has shown that apoptosis is an active process regulated by a series of proto-oncogenes and tumor suppressor genes associated with proliferation and differentiation, such as p53, Bcl-2, c-myc, ICE, Fas/APO-1 etc[24-27].
Of particulate interest is the Bcl-2 gene, which was discovered in 1984 through the cloning of the translocation breakpoint in nodular B cell lymphoma and located at 18q21. Later, the product of Bcl-2 was shown to be a mitochondrial internal membrane protein which had the unique property of blocking apoptosis induced in cultured immature B cells[28-30]. To date, the mechanisms of apoptosis blocking of Bcl-2 has been shown to be a complicated process, involving the regulation of a growing list of proteins, which share sequence homology with Bcl-2, and the relevant genes[31,32]. One of these proteins, Bax, opposes the action of Bcl-2 and promotes apoptosis. It has been proved that apoptosis has been regulated by the proportion of Bcl-2 and Bax. The Bax's homologous dimer can induce apoptosis, but the rising Bcl-2 protein combining with Bax will form Bax-Bcl-2 heterogenous dimer that is more stable than "Bax-Bax". This will neutralize the Bax function of inducing apoptosis[33,34]. Therefore, only high expression of Bcl-2 protein is not sure to inhibit apoptosis. Our results indicate that downregulation of Bcl-2 protein expression did not appear with the increase in apoptotic index for cells irradiated by X-rays and fast-neutron-rays.
The tumor suppressor gene p53 is indispensable for normal development. It plays a central role in the cellular response to DNA damage from both endogenous and exogenous sources, providing a protection against tumorigenesis. Activation of p53 may result in a cell cycle delay, presumably to allow an opportunity for DNA repair to occur before replication or mitosis[35,36]. In some cell types, however, p53 activation results in apoptotic cell death as a means of eliminating irreparably damaged cells. The final outcome of p53 activation depends on many factors, and is mediated largely through the action of downstream effector gene transactivated by p53[37]. In our experiment, high expression of mt p53 protein appeared in cells without radiation. After being radiated with various doses of X-rays, downregulation of mt p53 protein occurred. In contrast, downregulation of mt p53 protein has not appeared in cells irradiated by various doses of fast-neutron-rays. Some evidence has shown that the difference of DNA dsb (double strand break) repair area exist[38], which suggests basic differences in the nature of the lesions induced by high and low LET ionizing radiations. Other data has provided clear experimental evidence for the existence of clustered DNA double-strand breaks and demonstrate that short DNA fragments may be produced by neutron radiation, an observation not made for damage by low-LET radiation[39]. Additionally, It has been reported that the DNA damage by fast neutrons was shown to be significantly greater than for the same absorbed dose of X-rays[40]. On these grounds, we infer that X-rays and fast-neutron-rays may lead to the different DNA damage, e.g. a single or double strand break in DNA, nuclear supercoils, or the differences in the nature of dsb (double-strand break) induced and the way they are repaired. The difference of DNA damage may lead to different pathway of apoptosis involved in the different regulation of relevant apoptotic genes. Fast-neutron-rays may induce the involvement of apoptotic gene other than p53.