Published online Jun 15, 2001. doi: 10.3748/wjg.v7.i3.352
Revised: September 3, 2000
Accepted: September 19, 2000
Published online: June 15, 2001
AIM: To study the genetic alteration in ACF and to define the possibility that ACF may be a very early morphological lesion with molecular changes, and to explore the relationship between ACF and colorectal adenoma even carcinoma.
METHODS: DNA from 35 CRC, 15 adenomas, 34 ACF and 10 normal mucus was isolated by means of microdissection. Direct gene sequencing of K-ras gene including codon 12, 13 and 61 as well as the mutation cluster region (MCR) of APC gene was performed.
RESULTS: K-ras gene mutation frequency in ACF, adenoma and carcinoma was 17.6% (6/34), 13.3% (2/15), and 14.3% (5/35) respectively, showing no difference (P > 0.05) in K-ras gene mutation among three pathologic procedures. The K-ras gene mutation in adenoma, carcinoma and 4 ACF restricted in codon 12 (GGT→GAT), but the other 2 mutations from ACF located in codon 13 (GGC→GAC). K-ras gene mutation was found more frequently in older patients and patients with polypoid cancer. No mutation in codon 61 was found in the three tissue types. Mutation rate of APC gene in adenoma and carcinoma was 22.9% (8/35) and 26.7% (4/15), which was higher than ACF (2.9%) (P < 0.05). APC gene mutation in carcinoma was not correlated with age of patients, location, size and differentiation of tumor.
CONCLUSION: ACF might be a very early morphological lesion in the tumorogenesis of colorectal tumor. The morphological feature and gene mutation status was different in ACF and adenoma. ACF is possibly putative "microadenoma" that might be the precursor of adenoma. In addition, the development of a subgroup of colorectal carcinomas might undergo a way of "normal epithelium→ACF→carcinomas".
- Citation: Yuan P, Sun MH, Zhang JS, Zhu XZ, Shi DR. APC and K-ras gene mutation in aberrant crypt foci of human colon. World J Gastroenterol 2001; 7(3): 352-356
- URL: https://www.wjgnet.com/1007-9327/full/v7/i3/352.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i3.352
Colorectal cancer (CRC) is a complex pathological procedure in which multiple genes are involved during multiple steps. It is widely accepted that the order of "normal epithelium→hyperplastic epithelium→adenoma→cancer→cancer with metastasis" exists in majority of coloretal carcinoma[1,2]. The corresponding molecular order is demonstrated as follows: APC→altered methylation[3]→K-ras→MCC/DCC→P53. The activation of K-ras gene and inactivation of APC gene are frequent early events in the carcinogenesis of colorectal carcinoma[4-8]. Recent studies showed that aberrant crypt foci (ACF) is the earliest morphological lesion detectable in colorectal epithelium[9,10]. We studied CRC, adenoma and ACF with the aim to understand the relationship between ACF and colorectal neoplasm.
Thirty-five CRC were obtained from an unselected cohort in which the patients underwent initial curative resection in 1999 in the Cancer Hospital of Fudan University. Twenty CRC occurred in men and 15 in women. The age of the patients ranged from 28 to 85 years. The median age was 54.2. Fourteen of 35 patients had their tumor in proximal colon, and 21 in the distal part of large intestine (including rectum). Macroscopically, 22 were ulcerative type with more endophytic extension and 11 showed exophytic growth (including rape flower like polypoid and nodal). Histologically, 6 were highly, 22 were moderately and 7 were poorly differentiated. Fifteen adenomas during the same time period were collected from 9 men and 6 women. In histological type, 5 were tubular, 6 tubular villous, and 4 villous adenoma. The macroscopically normal epithelium 5 cm apart from the tumor site served as ACF study and that 10 cm at least apart from the primary tumor as normal control.
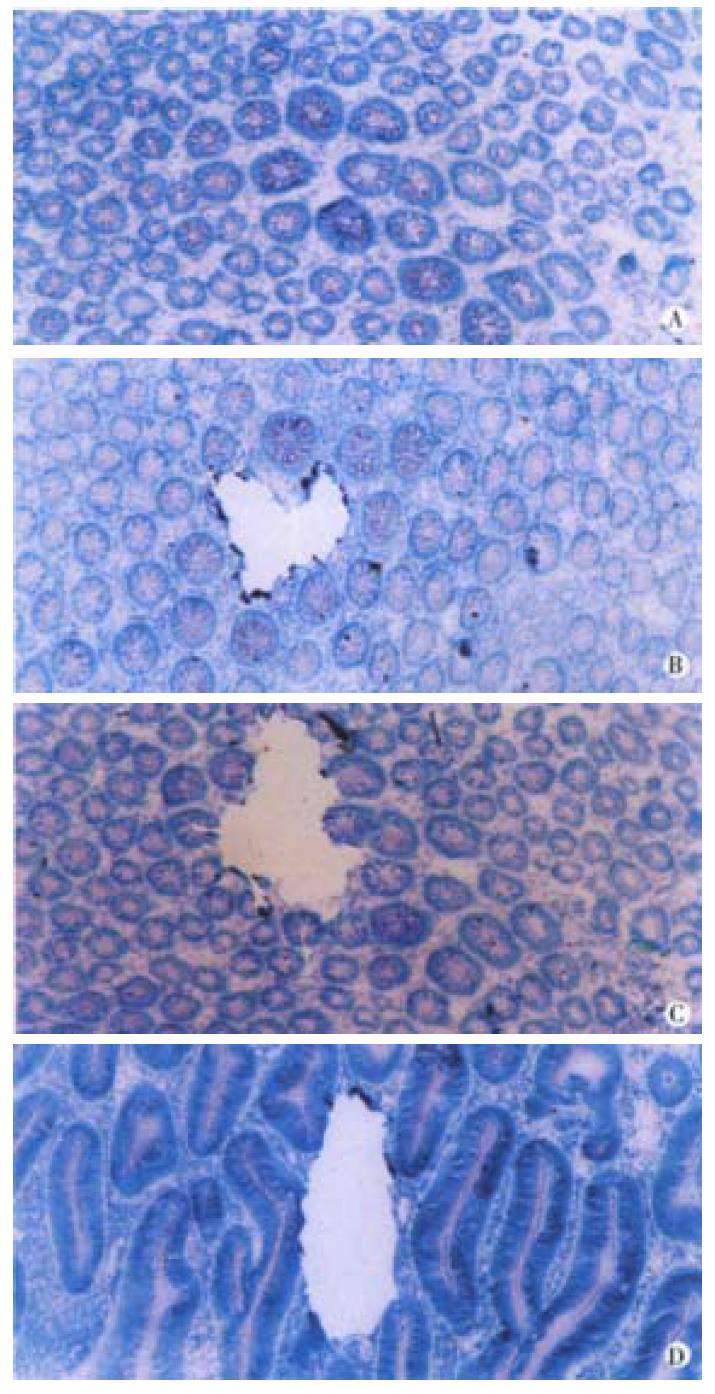
Cryostat frozen sections (5 μm, stained with 0.1% methylene blue for 5 min) of epithelium 5 cm apart from the primary tumor were used for identifying ACF according to Shpitz and Gregorio et al[11-15]. ACF was determined by two qualified pathologists when the following features appeared: large crypts, tight arranging, dark and overlapping nuclei, dysplastic morphology, "saw tooth like" and elongated luminal surface, follicular distribution and no inflammation cells or lymph follicle (Figure 1). Ten slides of 8 μm from 10 normal epithelia, 34 identified ACF and 35 CRC were stained with methylene blue and subsequently microdissected under the dissect microscope (40 folds). Individual crypts from CRC, adenoma, ACF and normal epithelium were isolated with scalpels and transferred to the centrifuge tube for DNA extraction. One crypt or about 100 cells from each case were used for the study[16] (Figure 1).
Microdissected tissue samples were digested in 50 μL cell lysis buffer (0.5 M Tris-Cl, pH8.9, 20 mM EDTA, 10 mM NaCl, 1% SDS), digested with proteinase K (500 mg/L) overnight at 37 °C. Genomic DNA was purified using DNA extract kit (DX Biotech Co. Ltd., Shanghai). The precipitation was suspended in TE for using[17,18].
Computer APC gene analysis software was used and according to the study by Losi[19-23]. The primers for K-ras gene encompassing exon 1, 2, the primers for APC gene encompassing the mutation cluster region in exon 15 (codon 1263-1596) were designed. PCR products were checked in agarose gel for size confirmation. All primers and PCR protocols are listed in Table 1.
| Genes | Region | Codon | Size of PCR | AT | Sequence |
| K-ras | exon1 | 1-54 | 163 | 56 °C | 5-GACTGAATATAAACTTGTGG |
| 5-CTGTATCAAAGAAGTGTCCT | |||||
| K-ras | exon2 | 31-84 | 161BP | 54 °C | 5-GACTGTGTTTCTCCCTTCT |
| 5-GGCAAATACACAAAGAAAG | |||||
| APC | 15-A | 1263-1393 | 390BP | 54 °C | 5-GTGTAGAAGATACTCCAATA |
| 5-GTGAACTGACAGAAGTACAT | |||||
| APC | 15-B | 1338-1436 | 295BP | 56 °C | 5-CAGGGTTCTAGTTTATCTTC |
| 5-TTCTGCTTGGTGGCATGGTT | |||||
| APC | 15-C | 1412-1515 | 310BP | 56 °C | 5-GGAATGGTAAGTGGCATAAT |
| 5-AAATGGCTCATCGAGGCTCA | |||||
| APC | 15-D | 1496-1596 | 300BP | 56 °C | 5-ACTCCAGATGGATTTTCTTG |
| 5-GGCTGGCTTTTTTGCTTTAC |
PCR products were purified with QIAquick PCR kit as described. Sequencing reaction was performed with Ready Reaction Kit from PE Company (96 °C10″→55 °C5″→60 °C4′,25 cycles). The reaction product was precipitated in 100% ethanol (2.5 volume) and 3 M NaAc (0.1 volume), and washed with 75% ethanol. The precipitation was resuspended in TSR (template suppression reagent), denatured at 94 °C and snap cooled at 4 °C before the automatic electrophoresis.
Chi-square study was used for the comparison between two groups.
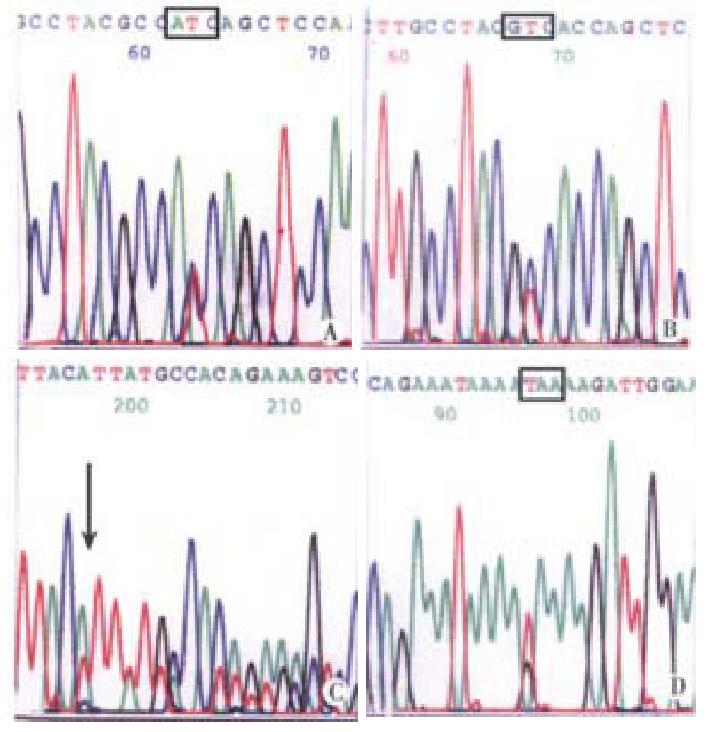
K-ras gene mutation and its feature-Five of 35 (14.3%) carcinomas, 2 of 15 (13.3%) adenomas and 6 of 34 (17.6%) ACF showed K-ras gene mutation. The mutation frequency was comparable among three types of tissues (P > 0.05). No mutation was detected in normal epithelium. The mutation in carcinoma, adenoma and 4 ACF located at the second nucleotide of codon 12 (GGT→GAT). Two mutation in ACF located at the second nucleotide of codon 13, (GGC→GAC). The carcinoma and ACF patient No. 30 shared the same mutation at codon 12. No mutation of codon 61 was found. K-ras mutation in carcinoma was related to the age and macroscopic type. The patients with mutation (median age of 70.8 years) were older than the patients without mutation (median age of 52.3 years) (P < 0.01). Four out 5 carcinomas with mutation were polypoid carcinoma (P < 0.05). All of 6 ACF with mutation were obtained from distal colon, most of their primary carcinomas showing polypoid (5 cases). The patients with mutation (median age of 68.4 years) in their ACF were older than that without mutation (median age of 43) in their ACF. This is coincident with the mutation in carcinomas. One of 2 adenomas with mutation was villous adenoma and another one was tubular adenoma (Figure 1).
Eight out of 35 carcinomas (22.9%) and 4 of 15 adenomas (26.7%) showed mutation in APC gene. The mutation frequency was close in two tissue types (P > 0.05). Only 1 of 34 ACF showed mutation. APC gene mutation in carcinoma was not related to the age, gender, tumor site, macroscopic type and histological differentiation. All 8 mutations were scattered in region A (2 cases), region B (4 cases), and in region C and D (1 case), respectively. Among the 4 cases of adenomas with APC mutation, 2 were villous adenomas and other two were villous tubular adenomas; 2 mutations in region A, 1 in region C and 1 in region D. A mutation in region A was demonstrated in both ACF and primary carcinoma (Figure 2). APC gene mutations were displayed in Table 2. Altogether 13 mutations were detected in regions A→D of APC gene, 5 were stop codon, 1 was nonsense mutation, the other were point mutation.
| DNA | Region | Codon | Mutation | Amino acid exchange |
| N 3 CRC | A | 1354 | TTT→TTA | Phe→Leu |
| N17 CRC | A | 1309 | GAA→TAA | Stop cocon |
| N14 CRC | B | 1389 | TCT→TTT | Ser→Phe |
| N 5 CRC | B | 1357 | GGA→AGA | Gly→Arg |
| N21 CRC | B | 1365 | GGT→GGC | nonsense |
| N26 CRC | B | 1398 | AGT→ACT | Ser→Thr |
| N28 CRC | C | 1465 | GTG→GCG | Val→Ala |
| N33 CRC | D | 1547 | GAA→TAA | Stop codon |
| Tubular villous adenoma | A | 1301 | ins1 A | Stop codon |
| Villous adenoma | A | 1309 | GAA→TAA | Stop codon |
| Villous adenoma | C | 1490 | ins8 TTATTACA | Frame shift |
| Tubular villous adenoma | B | 1367 | CAG→CAC | Gln→His |
| ACF | A | 1309 | GAA→TAA | Stop codon |
In 1987, Bird[24] established a mouse model of colorectal carcinoma by injecting F334 mouse with carcinogen AOM. He observed the changes of methylene blue stained large intestine mucus in different stages and found for the first time the earliest morphological change in normal colorectal mucus. Several or dozen aberrant crypts, scattering in the crypt level, were termed as aberrant crypt foci. It is recently reported that similar lesions exist in the human colorectal epithelium apart from the carcinoma[25-27]. The order "normal epithelium→metaplasia→adenoma→carcinoma→carcinoma with metastasis" is widely accepted by most authors. APC and K-ras gene mutation is the early event in the carcinogenesis[28-30]. Our results revealed that normal epithelium showed no mutation, but K-ras gene mutation appeared in ACF, adenoma and carcinoma with a close rate (P > 0.05), suggesting that K-ras mutation initiates in ACF stage and maintains during the process of the carcinogenesis. This demonstrates the possible relationship of ACF to carcinoma and ACF as a preneoplastic lesion in the carcinogenesis of colorectal carcinoma[31,32]. K-ras gene point mutation at codon 12 and 13 endows the epithelium with transformation ability. All mutations in carcinoma and adenoma located at codon 12 (4/6), while 1/3 mutation (2/6) of ACF was found at codon 13 (GGC→GAC). In the previous reports by American and European scientists, the K-ras gene mutation located both at codon 12 and 13, more frequently at codon 12[16,33-37]. Japanese and Chinese scientists reported the similar results as ours[22,38,39], the mutation in carcinoma limited in codon 12. The reason might be the difference in the genetic predisposition, the food, the environment and the pathogeny. The cell clone with mutation at codon 13 might have weaker clone selection and such cells have, therefore, weaker ability to expand themselves to grow out. ACF is reported to be located more frequently in rectum than in colon, more in distal than in proximal colon[40]. All 6 ACF with K-ras gene mutation in the current study located in distal colon, implying the same site of predisposition of colorectal carcinoma. ACF might be the earliest morphological lesion with detectable molecular genetic alteration in it.
APC gene was cloned, isolated and defined as a tumor suppressor gene in 1990. Germline mutation of the gene is responsible for the pathogenesis of familial adenomatous polyposis (FAP). Thirty-five sporadic CRC and 15 adenomas had similar frequent APC gene mutation in the MCR (P > 0.05). It is coincident with previous report[41-45]. APC gene mutation was not correlated with age, tumor site, macroscopic type and histologic differentiation. This is identical to the previous demonstration that APC gene is involved very early in the carcinogenesis of sporadic CRC. There have been many reports about the APC gene mutation in sporadic adenoma. APC gene mutation occurred even in the adenoma < 0.3 cm. The mutation was more frequently found in villous and tubular villous adenoma than in tubular adenoma. Our results support this documentation. Two of 4 adenomas with APC gene mutation were villous adenoma and 2 were tubular villous adenoma. The same mutation in codon 1309 (GAA→TAA) was found simultaneously in ACF, adenoma and primary carcinoma. This mutation was also found in other 4 of 15 adenomas. The mutation at codon 1309 is also the hot spot in Chinese sporadic colon tumors. Exactly alike the other reports, the mutation at codon 1301, 1309 and 1547 lead to stop codon. It is reported that the mutations cause most commonly truncation of the protein. The truncated protein binds to the wild type protein, causing a negative effect and decreasing their function as a tumor suppressor[46-48]. Because APC gene is too large to be wholly sequenced and the mutations scatter throughout over the gene, it is difficult for us with so less cases to find more mutation characterization of this gene.
ACF differ with adenoma in morphological and molecular level on the following points: ① ACF is surrounded by normal crypts, ② cells in ACF are not so dysplastic as that in adenoma, ③ mitosis is rare, ④ ACF is much smaller than adenoma, ⑤ APC mutation is rare, ⑥ mutation in K-ras gene at codon 13 is detectable. The above fact suggests the hypothesis that ACF is a preneoplastic lesion. The concept "microadenoma" could be used to describe such morphological lesion[49]. Nucci and Kobayashi have suggested that ACF could be added to the order: "normal epithelium→ACF→adenoma→carcinoma"[50-52]. Many studies demonstrated that K-ras gene mutation is induced by the APC gene mutation. We found in our study in contrast that 6/34 ACF displayed K-ras gene mutation. Only 1 out of 6 showed APC gene mutation. The possible interpretations are: ① not all K-ras gene mutations are induced by APC gene mutation, ② adenoma formation could occur in the basis of APC and K-ras gene mutation, ③ ACF formation is related to K-ras gene mutation but not to APC gene, ④ there might be another path of CRC. In addition, 27 of 34 CRC in our study were not accompanied with adenoma, but the ACF is proved to possess the same molecular alteration as in the primary CRC. We speculate that except the classical path of CRC, there might be another path "normal epithelium→ACF→carcinoma", without the stage of adenoma. More detailed molecular approach to understanding the role of ACF in this path is necessary.
Dr. Ping Yuan, Studying Province, studying in Medical College of Fudan University, worked in Department of Pathology, Wannan Medical College, having eighteen papers published.
This subject is supported by the Fund for Returned Scientists and Scholars, [1999] 363, Chinese Ministry of Education.
Edited by Ma JY
| 1. | Sedivy R, Wolf B, Kalipciyan M, Steger GG, Karner-Hanusch J, Mader RM. Genetic analysis of multiple synchronous lesions of the colon adenoma-carcinoma sequence. Br J Cancer. 2000;82:1276-1282. [PubMed] |
| 2. | Hoppe-Seyler F, Butz K. Tumor suppressor genes in molecular medicine. Clin Investig. 1994;72:619-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Zhang J, Lai MD, Chen J. Methylation status of p16 gene in colorectal carcinoma and normal colonic mucosa. World J Gastroenterol. 1999;5:451-454. [PubMed] |
| 4. | Peng DF, Lin HM. Expression of P53 oncoprotein in benign and malignant lesions of large bowel. China Natl J New Gastroenterol. 1996;2:236-237. |
| 5. | Fang DC, Luo YH, Lu R, Liu WW, Liu FX, Liang ZY. Loss of heterozygosity at APC, MCC and DCC genetic loci in colorectal cancers. China Natl J New Gastroenterol. 1995;1:21-24. |
| 6. | Zhao P, Hu YC, Wang DW, Wang ZP, Xu XZ, Yi PY, Gao YB, Yang GH. Relationship between loss of heterozygosity of microsatellite on DCC gene and prognosis of colorectal adenocarcinoma. China Natl J New Gastroenterol. 1997;3:121-122. |
| 7. | Chen DW, Wang YH, Chen XY, Wang Q, Gao H. Clinical significance of immunohistochemical study of P53 protein in colorectal carcinoma. China Natl J New Gastroenterol. 1996;2:25-26. |
| 8. | Scott N, Sagar P, Stewart J, Blair GE, Dixon MF, Quirke P. p53 in colorectal cancer: clinicopathological correlation and prognostic significance. Br J Cancer. 1991;63:317-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 174] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Zhang XM, Stamp D, Minkin S, Medline A, Corpet DE, Bruce WR, Archer MC. Promotion of aberrant crypt foci and cancer in rat colon by thermolyzed protein. J Natl Cancer Inst. 1992;84:1026-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Takahashi M, Minamoto T, Yamashita N, Yazawa K, Sugimura T, Esumi H. Reduction in formation and growth of 1,2-dimethylhydrazine-induced aberrant crypt foci in rat colon by docosahexaenoic acid. Cancer Res. 1993;53:2786-2789. [PubMed] |
| 11. | Shpitz B, Bomstein Y, Mekori Y, Cohen R, Kaufman Z, Neufeld D, Galkin M, Bernheim J. Aberrant crypt foci in human colons: distribution and histomorphologic characteristics. Hum Pathol. 1998;29:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Di Gregorio C, Losi L, Fante R, Modica S, Ghidoni M, Pedroni M, Tamassia MG, Gafà L, Ponz de Leon M, Roncucci L. Histology of aberrant crypt foci in the human colon. Histopathology. 1997;30:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Shpitz B, Hay K, Medline A, Bruce WR, Bull SB, Gallinger S, Stern H. Natural history of aberrant crypt foci. A surgical approach. Dis Colon Rectum. 1996;39:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Nucci MR, Robinson CR, Longo P, Campbell P, Hamilton SR. Phenotypic and genotypic characteristics of aberrant crypt foci in human colorectal mucosa. Hum Pathol. 1997;28:1396-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Kristt D, Bryan K, Gal R. Colonic aberrant crypts may originate from impaired fissioning: relevance to increased risk of neoplasia. Hum Pathol. 1999;30:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Sun MH, Gebert J, Riedl S, von Knebel Doeberitz M. Early molecular biological events of the adenomas from familial adenomatous polyposis: analysis of single crypt microdissection. Zhongguo Aizheng Zazhi. 2000;10:7-10. |
| 17. | Gebert J, Sun M, Ridder R, Hinz U, Lehnert T, Möller P, Schackert HK, Herfarth C, von Knebel Doeberitz M. Molecular profiling of sporadic colorectal tumors by microsatellite analysis. Int J Oncol. 2000;16:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Wang L, Lu W, Chen YG, Zhou XM, Gu JR. Comparison of gene expression between normal colon mucosa and colon carcinoma by means of messenger RNA differential display. World J Gastroenterol. 1999;5:533-534. [PubMed] |
| 19. | Losi L, Roncucci L, di Gregorio C, de Leon MP, Benhattar J. K-ras and p53 mutations in human colorectal aberrant crypt foci. J Pathol. 1996;178:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Sowa M, Nakata B. [Genome analyses for precancerous lesions in the gastrointestinal tract]. Gan To Kagaku Ryoho. 2000;27:335-340. [PubMed] |
| 21. | Chiang JM, Chou YH, Chou TB. K-ras codon 12 mutation determines the polypoid growth of colorectral cancer. Cancer Res. 1998;58:3289-3293. [PubMed] |
| 22. | Lee JC, Wang ST, Lai MD, Lin YJ, Yang HB. K-ras gene mutation is a useful predictor of the survival of early stage colorectal cancers. Anticancer Res. 1996;16:3839-3844. [PubMed] |
| 23. | Mulkens J, Poncin J, Arends JW, De Goeij AF. APC mutations in human colorectal adenomas: analysis of the mutation cluster region with temperature gradient gel electrophoresis and clinicopathological features. J Pathol. 1998;185:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 726] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 25. | Olivo S, Wargovich MJ. Inhibition of aberrant crypt foci by chemopreventive agents. In Vivo. 1998;12:159-166. [PubMed] |
| 26. | Pretlow TP, Barrow BJ, Ashton WS, O'Riordan MA, Pretlow TG, Jurcisek JA, Stellato TA. Aberrant crypts: putative preneoplastic foci in human colonic mucosa. Cancer Res. 1991;51:1564-1567. [PubMed] |
| 27. | Bouzourene H, Chaubert P, Seelentag W, Bosman FT, Saraga E. Aberrant crypt foci in patients with neoplastic and nonneoplastic colonic disease. Hum Pathol. 1999;30:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 29. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 7997] [Article Influence: 228.5] [Reference Citation Analysis (1)] |
| 30. | Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1301] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 31. | Luceri C, De Filippo C, Caderni G, Gambacciani L, Salvadori M, Giannini A, Dolara P. Detection of somatic DNA alterations in azoxymethane-induced F344 rat colon tumors by random amplified polymorphic DNA analysis. Carcinogenesis. 2000;21:1753-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Spandidos DA, Glarakis IS, Kotsinas A, Ergazaki M, Kiaris H. Ras oncogene activation in benign and malignant colorectal tumours. Tumori. 1995;81:7-11. [PubMed] |
| 34. | Zhang H, Nordenskjöld B, Dufmats M, Söderkvist P, Sun XF. K-ras mutations in colorectal adenocarcinomas and neighbouring transitional mucosa. Eur J Cancer. 1998;34:2053-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Halatsch ME, Hirsch-Ernst KI, Weinel RJ, Kahl GF. Differential activation of the c-Ki-ras-2 proto-oncogene in human colorectal carcinoma. Anticancer Res. 1998;18:2323-2325. [PubMed] |
| 36. | Tang WY, Elnatan J, Lee YS, Goh HS, Smith DR. c-Ki-ras mutations in colorectal adenocarcinomas from a country with a rapidly changing colorectal cancer incidence. Br J Cancer. 1999;81:237-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Andreyev HJ, Tilsed JV, Cunningham D, Sampson SA, Norman AR, Schneider HJ, Clarke PA. K-ras mutations in patients with early colorectal cancers. Gut. 1997;41:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Yamashita N, Minamoto T, Ochiai A, Onda M, Esumi H. Frequent and characteristic K-ras activation in aberrant crypt foci of colon. Is there preference among K-ras mutants for malignant progression. Cancer. 1995;75:1527-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 39. | Ajioka Y, Watanabe H, Jass JR, Yokota Y, Kobayashi M, Nishikura K. Infrequent K-ras codon 12 mutation in serrated adenomas of human colorectum. Gut. 1998;42:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Roncucci L, Modica S, Pedroni M, Tamassia MG, Ghidoni M, Losi L, Fante R, Di Gregorio C, Manenti A, Gafa L. Aberrant crypt foci in patients with colorectal cancer. Br J Cancer. 1998;77:2343-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Smith AJ, Stern HS, Penner M, Hay K, Mitri A, Bapat BV, Gallinger S. Somatic APC and K-ras codon 12 mutations in aberrant crypt foci from human colons. Cancer Res. 1994;54:5527-5530. [PubMed] |
| 42. | Poncin J, Mulkens J, Arends JW, de Goeij A. Optimizing the APC gene mutation analysis in archival colorectal tumor tissue. Diagn Mol Pathol. 1999;8:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Prior TW, Chadwick RB, Papp AC, Arcot AN, Isa AM, Pearl DK, Stemmermann G, Percesepe A, Loukola A, Aaltonen LA. The I1307K polymorphism of the APC gene in colorectal cancer. Gastroenterology. 1999;116:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Gryfe R, Di Nicola N, Gallinger S, Redston M. Somatic instability of the APC I1307K allele in colorectal neoplasia. Cancer Res. 1998;58:4040-4043. [PubMed] |
| 45. | De Filippo C, Caderni G, Bazzicalupo M, Briani C, Giannini A, Fazi M, Dolara P. Mutations of the APC gene in experimental colorectal carcinogenesis induced by azoxymethane in F344 rats. Br J Cancer. 1998;77:2148-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | De Benedetti L, Sciallero S, Gismondi V, James R, Bafico A, Biticchi R, Masetti E, Bonelli L, Heouaine A, Picasso M. Association of APC gene mutations and histological characteristics of colorectal adenomas. Cancer Res. 1994;54:3553-3556. [PubMed] |
| 47. | Bjerknes M, Cheng H, Hay K, Gallinger S. APC mutation and the crypt cycle in murine and human intestine. Am J Pathol. 1997;150:833-839. [PubMed] |
| 48. | Otori K, Konishi M, Sugiyama K, Hasebe T, Shimoda T, Kikuchi-Yanoshita R, Mukai K, Fukushima S, Miyaki M, Esumi H. Infrequent somatic mutation of the adenomatous polyposis coli gene in aberrant crypt foci of human colon tissue. Cancer. 1998;83:896-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Katsuki S, Oui M, Takayama T, Takahashi Y, Shuichi N, Niitsu Y. [Aberrant crypt foci as biomarkers in chemoprevention for colorectal cancer]. Nihon Geka Gakkai Zasshi. 1998;99:379-384. [PubMed] |
| 50. | Zhu D, Keohavong P, Finkelstein SD, Swalsky P, Bakker A, Weissfeld J, Srivastava S, Whiteside TL. K-ras gene mutations in normal colorectal tissues from K-ras mutation-positive colorectal cancer patients. Cancer Res. 1997;57:2485-2492. [PubMed] |
| 51. | Kobayashi M, Watanabe H, Ajioka Y, Honma T, Asakura H. Effect of K-ras mutation on morphogenesis of colorectal adenomas and early cancers: relationship to distribution of proliferating cells. Hum Pathol. 1996;27:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Shivapurkar N, Huang L, Ruggeri B, Swalsky PA, Bakker A, Finkelstein S, Frost A, Silverberg S. K-ras and p53 mutations in aberrant crypt foci and colonic tumors from colon cancer patients. Cancer Lett. 1997;115:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |