Published online Dec 15, 2000. doi: 10.3748/wjg.v6.i6.824
Revised: June 19, 2000
Accepted: June 29, 2000
Published online: December 15, 2000
AIM: To assess the effect of ACE inhibitor and AngII type 1 (AT1) receptor antagonist in preventing hepatic fibrosis caused by CCl4 administration in rats; to investigate whether or not there are expression of AT1 receptors on hepatic stellate cells; and to observe the effect of AngII on proliferation and ECM synthesis of cultured HSCs.
METHODS: Studies were conducted in male Sprague-Dawley rats. Except for the hepatofibrotic model group and the control group, in three treated groups, either enalapril (5 mg/kg), or losartan (10 mg/kg), or enalapril + losartan were given to the fibrotic rats by daily gavage, and saline vehicle was given to model and normal control rats. After 6 wk, liver fibrosis was assessed directly by hepatic morphometric analysis, which has been considered the gold standard for the quantification of fibrosis. The expressions of AT1 receptors and (α-mooth muscle actin, α-SMA) in liver tissue or isolated hepatic stellate cells (HSCs) were detected by immunohistochemical techniques. The effect of AngII on HSC proliferation was determined by MTT method. Effect of AngII on collagen synthesis of HSCs was de termined by 3H-proline incorporation.
RESULTS: Contrasted to the fibrosis in rats of the model group, groups of rats treated with either enalapril or losartan, or a combination of two drugs showed a limited expansion of the interstitium (4.23 ± 3.70 vs 11.22 ± 4.79, P < 0.05), but no difference was observed among three treated groups (5.38 ± 3.43, 4. 96 ± 2.96, 4.23 ± 2.70, P > 0.05). Expression of AT1 receptors was found in fibrotic interstitium of fibrotic rats, whereas in normal control rats they were limited to vasculature only to a very slight degree. AT1 receptors were also expressed on activated HSCs in the culture. At concentrations from 10-9 to 10-5 mol/L, AngII stimulated HSC proliferation in culture in a dose-dependent manner. Increasing AngII concentrations produced corresponding increases in 3H-proline incorporation. Differences among groups were significant.
CONCLUSION: Angiotensin-converting enzyme inhibitors and AT 1 blocker may slow the progression of hepatic fibrosis; activated HSCs express AT1 receptors, and AngII can stimulate the proliferation and collagen synthesis of HSCs in a dose-dependent manner; and activation of RAS may be related to hepatic fibrogenesis induced by CCl4.
- Citation: Wei HS, Lu HM, Li DG, Zhan YT, Wang ZR, Huang X, Cheng JL, Xu QF. The regulatory role of AT1 receptor on activated HSCs in hepatic fibrogenesis: Effects of RAS inhibitors on hepatic fibrosis induced by CCl4. World J Gastroenterol 2000; 6(6): 824-828
- URL: https://www.wjgnet.com/1007-9327/full/v6/i6/824.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i6.824
Liver fibrosis is a conseqence of chronic liver injury from different causes, including alcohol, toxins, chronic viral infections, metabolic disease, etc. In liver fibrogenesis there is an increased deposition of extracellular matrix (ECM) in the perisinusoidal and periportal spaces. The activated hepatic stellate cells (HSCs), have now been identified as the primary source of ECM synthesis in liver fibrogenesis[1-3]. The pathogenetic significance of HSC relies on their ability to be activated into myofibroblast-like cells with enhanced production of ECM[4,5]. Proliferative cytokine like platelet-derived growth factor and fibrogenetic cytokines like transforming growth factor-β (TGF-β) are major cytokines involved in the activation process, causing enhanced proliferation of HSC and matrix synthesis[6,7].
Researches increasingly show that locally synthesized angiotensin II (AngII), as a fibrogenetic factor, is involved in cardiac fibrosis[8,9], renal interstitial fibrosis[10,11], and pulmonary fibrosis[12,13]. Thus, treatment with angiotensin-converting enzyme (ACE) inhibitor or angiotens in II type 1 (AT1) receptor antagonist may attenuate the cardiac fibrosis that occurrs in experimental myocardial infartion[8], and may also retard the progression of renal glomerulosclerosis and interstitial fibrosis[14,15] and pulmonary fibrosis[16].
It is believed that AngII has a direct fibrogenetic effect, independent of its systemic hemodynamic effect. The evidence supporting this notion comes from in vitro studies showing that the AT1 receptor is present on interstitial fi broblasts, such as cardiac fibroblasts[17], renal intersitial fibroblasts[18], and that AngII directly increases ECM synthesis in cultured fibroblasts.
We hypothesized that the activation of local RAS might also be related to hepatic fibrogenesis. Thus, it is important to ascertain the existence of expression AT1 receptor on HSCs in the process of hepatic fibrosis. If this hypothesis is true, new preventive and therapeutic approaches may be provided to hepatic fibrosis. This study was designed: to assess effects of ACE inhibitor, enalapril, and AT1 receptor antagonist, losartan, on hepatic fibrosis induced by CCl4; and to investigate whether or not there is expression of AT1 receptors on HSCs.
Adult male Sprague-Dawley rats (Animal Centre of Shanghai Medical University, China) weighing 180 g-200 g were housed in temperature and light-controlled room. The rats were divided into five groups (n = 10 in each group): the control group, model group and three treated groups. Except for rats of the control group, all rats were given subcutaneous injection of 40% CCl4 (CCl4∶olive oil 2∶3), 0.3 mL/100 g, every 3 d for 6 wk). Simultaneous treatments with enalapril (Changzhou Pharmacy, China) (5 mg·kg-1·d-1), losartan (Merk Co. England) (10 mg·kg-1·d-1), or enalapril + losartan administration (once a day by gavage for 6 wk) were withdrawn a day before the rats were killed for study.
After sacrificing the rats, blood samples were immediately taken and centrifuged at 4 °C, and plasma were kept at -20 °C for assays. The aspartate transaminase (AST) and alanine transaminase (ALT) activities were determined by the standard enzymatic methods. The serum levels of angiotensin II and renin activity were determined by radioimmunoassays (kit purchased from Northern Biot Co, China).
Liver fibrosis was assessed directly by hepatic morphometric analysis, which has been considered to be the gold standard for the quantification of fibrosis. For image analysis, three liver fragments (> 10 mm2) were randomly taken in the right, median, and left lobes of each rat liver. The liver sections were fixed in a 10% solution of formaldehyde in 0.1 mol/L phosphate-buffered saline (pH7.4), and embedded in paraffin. Five-micrometer slides were prepared. Collagen expression was detected with standard van Gieson staining. The histomorphormetric analysis was performed on a KS400 image analysis system (German). The liver slides were placed on the X-Y motorized stage of microscope after equalization of light intensity. The percentage of fibrosis or area of fibrosis could be obtained in microscopic fields. Total liver area of fibrosis was expressed as the mean of the percentage of fibrosis in the three liver fragments.
Immunohistochemical methods were used to detect the expressions of α-SMA and AT 1 (anti-rat rabit polyclonal AT 1 antibody was the product of Santa Cruz Biot Co, USA) receptors in liver tissue.
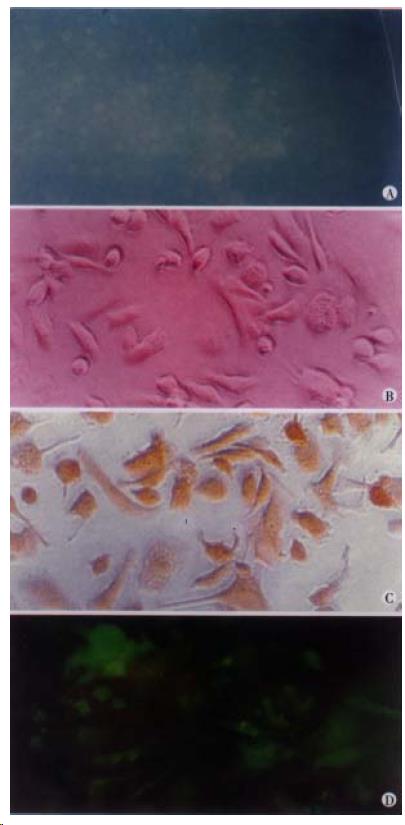
HSCs were isolated from normal male Sprague-Dawley rat (500 g-600 g body weight) liver by a combination of pronase-collagenase perfusion and density gradient contrifugation, as previously described[19], and identified by positive ultraviolet autofluorescence. Primary HSCs were cultured in 24-well plastic culture plate at a density of 5 × 105 cells/L, and maintained in Dulbecco’s modified Eagle medium (DMEM) with 15% fetal calf serum (FCS) (Sigma) and penicillin/streptomycin. After seven days, the cells were was hed with PBS, and the expression of α-SMA (Monoclonal antibody purchased from Maixin Biot Co. China) was determined by immunohistochemical method. The expression of AT1 receptor was detected by immunofluorescence staining with anti-rat rabbit polyclonal antibody (Santa Cruz Biot) and labeled with fluorescein-isothiocyanate (FITC, Sigma).
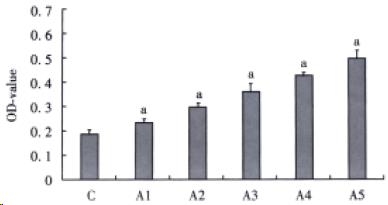
The effect of AngII on HSC proliferation was determined by MTT method. After being cultured for 4 d, HSCs were digested with 0.25% trypsin and then cultured in serum-free medium in 96-well culture plate (200 μL per well), to which was added AngII (to make a series of final concentrations: 10-9, 10-8, 10-7, 10-6, 10-5 mol/L). Each concentration included six wells, while the serum-free medium served as a control. After being cultured for 48 h, HSCs were supplemented with 20 μL MTT (5 g/L) (Fluka Co. Product) and incubated for another 6 h. Then the supernatant was discarded by aspiration and the HSC preparation was shaked with 200 μL DMSO for 10 min, before the OD value was measured at 490 nm.
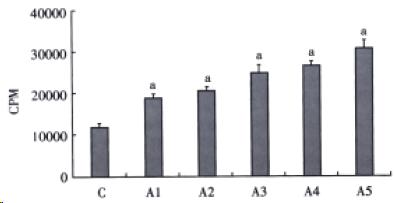
Collagen synthesis was determined by 3H-proline incorporation. After cultured for 48 h, HSCs were further cultured for additional 48 h in serum -free medium containing 2 μL 3H-proline in various concentrations of AngII (the final concentrations being 10-9, 10-8, 10-7, 10-6, 10-5 mol/L). Subsequently, HSCs were digested with 0.25% trypsin and collected on glass fiber membrane, then washed with PBS. After being dried, HSCs were suspended in 10 mL scintillation solution for 12 h. The radioactivity was determined by liquid scintillation and expressed as cpm/2.5 × 105 cells.
Data were presented as mean ± SEM. Comparisons among three or more groups were made by one-way ANOVA followed by Dunnett’s t test. A value of P < 0. 05 was considerd to be statistically significant.
Effects of RAS inhibitors on rat serum levels of ALT and AST Contrasted with rats of the control group, as shown in Table 1, rats receiving CCl4 had increased serum ALT and AST significantly. However, chronic administration of enalapril and losartan prevented the increase in ALT and AST (P < 0.01, Table 1).
Serum renin activity and AngII levels Serum renin activity (μg·L-1/h) of rats in the model group was three times higher than that of the control group (P < 0.01). Serum AngII (ng/L) levels of model group also increased two times than that of control group (P < 0.01). Treatment with RAS inhibitors significantly decreased the serum renin activity and serum levels of AngII (P < 0.05, Table 2).
Fibrosis quantification As expected, there was an increase in the area of fibrosis in rats of the model group compared with that of the control group. There was a significant decrease of fibrosis in all three treated groups (P < 0.0 5), but no significantly difference was observed between the enalapril and losartan treated groups (P > 0.05, Table 3).
Immunohistochemistry Expressions of AT1 receptors and α-SMA in liver tissue were detected by immunohistochemical methods. AT1 receptors mainly localized in the vasculature of liver tissue in the normal control rats. In rats of the model group, the expression of AT1 receptors mainly localized in the fibrotic areas, correlated with the expression of α-SMA. Enalapril, losartan, and combined treated groups showed a reduced AT1 receptor staining.
After culturing HSCs for seven days, the expression of AT1 receptors in HSC could be detected by FITC-immunofluorence, together with the expression of α-SMA (Figure 1A, Figure 1D).
Over a wide range of concentrations from 10-9 to 10-5 mol/L, AngII stimulated cultured HSCs proliferation in a dose-dependent manner (Figure 2). Increasing AngII concentration produced an increase in 3H-proline incorporation (Figure 3). Differences among groups were significant (P < 0.05).
The present study demonstrates for the first time the expression of AT1 receptor subtype in fibrotic liver tissue and in primary cultured rat HSC.
In recent years, a series of investigations demonstrated that fibrogenesis was related to the activation of local RAS[20-24]. Expression of AT1 receptors in several types of intersititial cells, such as cardiac fibroblasts[17], renal intersititial fibroblasts[18] and lung fibroblasts[25]. In vitro study suggested that AngII induced a net stimulation of collagen synthesis and expression of TGF-βvia AT1 receptors in a dose-dependent manner[26-30]. Blockade of AT1 receptors has been shown to inhibit DNA and collagen syntheses in intersititial cells after myocardial infarction[31,32], renal and pulmonary injury[15,33], and retarded the progression of cardiac, renal and pulmonary interstiti al fibrosis[34-41].
It is believed that the activation and proliferation of HSCs are major proce sses in hepatic fibrosis, although its pathogenesis has not been fully clarified [42-45]. The expression of AT1 receptors in HSCs and fibrotic liver tis sue supports the notion that activation of local RAS might be related to hepatic fibrogenesis induced by CCl4. Our results showed that in rat livers, the expression of AT1 receptors in fibrotic areas correlated with the expression of α-SMA. In primary cultured HSCs, the expression of AT1 receptors also correlated with α-SMA. These findings indicated that, as a growth factor of interstitialcells, AngII might prompt activation of HSCs in the process of hepatic fibro sis. Although both ACE inhibitor and AT1 receptor antagonist could alleviate hepatic fibrosis, as our results showed, a combination of these two gave no added effects of slowing the progression of hepatic fibrosis.
Another interesting finding in our study was that ACE inhibitor and AT1 receptor antagonist could attenuate the hepatocytic injury induced by CCl4, suggesting their cytoprotective effects by virtue of antifibrogenesis.
As the principal effector molecule of RAS, the production of a profibrogenic cytokine, TGF-β was enhanced by AngII in progressive fibrosis of heart and kidney. Recently, Powell et al[46]. demonstrated that a statistically significant relationship was observed between inheritance of high TGF-β and angiotensinogen-producing genotype and the development of progressive hepatic fibrosis. Patients with chronic hepatitis C virus infection who inherited neither of the profibrogenic genotype had no or only minimal fibrosis. The documentation of a significant relationship between angiotensinogen genotype and fibrosis also suggested that AngII might be another mediator of ECM production in the liver.
In conclusion, our investigation demonstrated that: ① ACE inhibitor and AT1 receptor antagonist might slow the progression of hepatic fibrosis induced by CCl4, but a combination of the two gave no additive effect; ② activated HSCs expressed AT1 receptors, and AngII could stimulate the proliferation and collagen synthesis of HSCs in a dose-dependent manner; and ③ local activation of R AS in liver tissue might be related to hepatic fibrogenesis induced by CCl4.
Edited by Lu HM
Proofread by Ma JY
| 1. | Gao ZL, Li DG, Lu HM, Gu XH. The effect of retinoic acid on Ito cell proliferation and content of DNA and RNA. World J Gastroenterol. 1999;5:443-444. [PubMed] |
| 2. | Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I, III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397-403. [PubMed] |
| 3. | Yin F, Yao SK, Li ZH, Shi HC. Study on the therapeutic effect of all-trans retinoic acid and malotilate on CCl4-induced hepatic fibrosis of rats. World J Gastroenterol. 1998;4:99. |
| 4. | Liu P, Liu C, Xu LM, Hu YY, Xue HM, Liu CH, Zhang ZQ. Effects of Fuzheng Huayu 319 recipe on liver fibrosis in chronic hepatitis B. World J Gastroenterol. 1998;4:348-353. [PubMed] |
| 5. | Cai DY, Zhao G, Chen JC, Ye GM, Bing FH, Fan BW. Therapeutic effect of Zijin capsule in liver fibrosis in rats. World J Gastroenterol. 1998;4:260-263. [PubMed] |
| 6. | Wang YF, Li QF, Wang H, Mao Q, Wu CQ. Effects of vitamin E on experimental hepatic fibrosis in rats. World J Gastroenterol. 1998;4:157. |
| 7. | Wang X, Chen YX, Xu CF, Zhao GN, Huang YX, Wang QL. Relationship between tumor necrosis factor-alphaand liver fibrosis. World J Gastroenterol. 1998;4:18. [PubMed] |
| 8. | Silvestre JS, Heymes C, Oubénaïssa A, Robert V, Aupetit-Faisant B, Carayon A, Swynghedauw B, Delcayre C. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation. 1999;99:2694-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 249] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Harada K, Sugaya T, Murakami K, Yazaki Y, Komuro I. Angiotensin II type 1A receptor knockout mice display less left ventricular remodeling and improved survival after myocardial infarction. Circulation. 1999;100:2093-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Metzger R, Bohle RM, Pauls K, Eichner G, Alhenc-Gelas F, Danilov SM, Franke FE. Angiotensin-converting enzyme in non-neoplastic kidney diseases. Kidney Int. 1999;56:1442-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Wolf G, Ziyadeh FN, Stahl RA. Angiotensin II stimulates expression of transforming growth factor beta receptor type II in cultured mouse proximal tubular cells. J Mol Med (. Berl). 1999;77:556-564. [PubMed] |
| 12. | Wang R, Ramos C, Joshi I, Zagariya A, Pardo A, Selman M, Uhal BD. Human lung myofibroblast-derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. Am J Physiol. 1999;277:L1158-L1164. [PubMed] |
| 13. | Wang R, Zagariya A, Ang E, Ibarra-Sunga O, Uhal BD. Fas-induced apoptosis of alveolar epithelial cells requires ANG II generation and receptor interaction. Am J Physiol. 1999;277:L1245-L1250. [PubMed] |
| 14. | Hebert LA, Falkenhain ME, Nahman NS, Cosio FG, O'Dorisio TM. Combination ACE inhibitor and angiotensin II receptor antagonist therapy in diabetic nephropathy. Am J Nephrol. 1999;19:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Hisada Y, Sakurai H, Sugaya T. Cell to cell interaction between mesangial cells and macrophages induces the expression of monocyte chemoattractant protein-1 through nuclear factor-kappaB activation. Biochem Biophys Res Commun. 2000;269:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Uhal BD, Gidea C, Bargout R, Bifero A, Ibarra-Sunga O, Papp M, Flynn K, Filippatos G. Captopril inhibits apoptosis in human lung epithelial cells: a potential antifibrotic mechanism. Am J Physiol. 1998;275:L1013-L1017. [PubMed] |
| 17. | Hafizi S, Wharton J, Morgan K, Allen SP, Chester AH, Catravas JD, Polak JM, Yacoub MH. Expression of functional angiotensin-converting enzyme and AT1 receptors in cultured human cardiac fibroblasts. Circulation. 1998;98:2553-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Hisada Y, Sugaya T, Yamanouchi M, Uchida H, Fujimura H, Sakurai H, Fukamizu A, Murakami K. Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest. 1999;103:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Huang ZG, Zhai WR, Zhang YE, Zhang XR. Study of heteroserum-induced rat liver fibrosis model and its mechanism. World J Gastroenterol. 1998;4:206-209. [PubMed] |
| 20. | Brilla CG. Renin-angiotensin-aldosterone system and myocardial fibrosis. Cardiovasc Res. 2000;47:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Pagtalunan ME, Olson JL, Meyer TW. Contribution of angiotensin II to late renal injury after acute ischemia. J Am Soc Nephrol. 2000;11:1278-1286. [PubMed] |
| 22. | Sun Y, Zhang J, Zhang JQ, Ramires FJ. Local angiotensin II and transforming growth factor-beta1 in renal fibrosis of rats. Hypertension. 2000;35:1078-1084. [PubMed] |
| 23. | Schnee JM, Hsueh WA. Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc Res. 2000;46:264-268. [PubMed] |
| 24. | Chatziantoniou C, Dussaule JC. Endothelin and renal vascular fibrosis: of mice and men. Curr Opin Nephrol Hypertens. 2000;9:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med. 2000;161:1999-2004. [PubMed] |
| 26. | Yoo KH, Thornhill BA, Wolstenholme JT, Chevalier RL. Tissue-specific regulation of growth factors and clusterin by angiotensin II. Am J Hypertens. 1998;11:715-722. [PubMed] |
| 27. | Grohé C, Kahlert S, Löbbert K, Neyses L, van Eickels M, Stimpel M, Vetter H. Angiotensin converting enzyme inhibition modulates cardiac fibroblast growth. J Hypertens. 1998;16:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Sano H, Okamoto H, Kitabatake A, Iizuka K, Murakami T, Kawaguchi H. Increased mRNA expression of cardiac renin-angiotensin system and collagen synthesis in spontaneously hypertensive rats. Mol Cell Biochem. 1998;178:51-58. [PubMed] |
| 29. | Kawano H, Do YS, Kawano Y, Starnes V, Barr M, Law RE, Hsueh WA. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation. 2000;101:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Kashiwagi M, Shinozaki M, Hirakata H, Tamaki K, Hirano T, Tokumoto M, Goto H, Okuda S, Fujishima M. Locally activated renin-angiotensin system associated with TGF-beta1 as a major factor for renal injury induced by chronic inhibition of nitric oxide synthase in rats. J Am Soc Nephrol. 2000;11:616-624. [PubMed] |
| 31. | Ju H, Zhao S, Jassal DS, Dixon IM. Effect of AT1 receptor blockade on cardiac collagen remodeling after myocardial infarction. Cardiovasc Res. 1997;35:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Ryoke T, Gu Y, Mao L, Hongo M, Clark RG, Peterson KL, Ross J. Progressive cardiac dysfunction and fibrosis in the cardiomyopathic hamster and effects of growth hormone and angiotensin-converting enzyme inhibition. Circulation. 1999;100:1734-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181-188. [PubMed] |
| 34. | Varo N, Iraburu MJ, Varela M, López B, Etayo JC, Díez J. Chronic AT (1) blockade stimulates extracellular collagen type I degradation and reverses myocardial fibrosis in spontaneously hypertensive rats. Hypertension. 2000;35:1197-1202. [PubMed] |
| 35. | Crenshaw G, Bigler S, Salem M, Crook ED. Focal segmental glomerulosclerosis in African Americans: effects of steroids and angiotensin converting enzyme inhibitors. Am J Med Sci. 2000;319:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Farina NK, Johnston CI, Burrell LM. Reversal of cardiac hypertrophy and fibrosis by S21402, a dual inhibitor of neutral endopeptidase and angiotensin converting enzyme in SHRs. J Hypertens. 2000;18:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Molteni A, Moulder JE, Cohen EF, Ward WF, Fish BL, Taylor JM, Wolfe LF, Brizio-Molteni L, Veno P. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Ikeda Y, Nakamura T, Takano H, Kimura H, Obata JE, Takeda S, Hata A, Shido K, Mochizuki S, Yoshida Y. Angiotensin II-induced cardiomyocyte hypertrophy and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. J Lab Clin Med. 2000;135:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257-263. [PubMed] |
| 40. | Border WA, Noble NA. Effect of maximal reduction of angiotensin in renal fibrosis: bad news-good news from a pediatric mouse. Am J Kidney Dis. 2000;35:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Katoh M, Egashira K, Mitsui T, Chishima S, Takeshita A, Narita H. Angiotensin-converting enzyme inhibitor prevents plasminogen activator inhibitor-1 expression in a rat model with cardiovascular remodeling induced by chronic inhibition of nitric oxide synthesis. J Mol Cell Cardiol. 2000;32:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Wei HS, Li DG, Lu HM, Zhan YT, Wang ZR, Huang X, Zhang J, Cheng JL, Xu QF. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl (4). World J Gastroenterol. 2000;6:540-545. [PubMed] |
| 43. | Liu CH, Liu C, Liu P, Xu LM. Seropharmalogical effects of Fuzheng Huayu decoction on rat Ito cell morphology and functions in culture. China Natl J New Gastroenterol. 1997;3:263-265. |
| 44. | Wang YJ, Sun ZQ, Quan QZ, Yu JJ. Fat storing cells and liver fibrosis. China Natl J New Gastroenterol. 1996;2:58-60. |
| 45. | Huang ZG, Zhai WR, Zhang YE, Zhang XR. Study of heteroserum-induced rat liver fibrosis model and its mechanism. World J Gastroenterol. 1998;4:206-209. [PubMed] |
| 46. | Powell EE, Edwards-Smith CJ, Hay JL, Clouston AD, Crawford DH, Shorthouse C, Purdie DM, Jonsson JR. Host genetic factors influence disease progression in chronic hepatitis C. Hepatology. 2000;31:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 304] [Article Influence: 12.2] [Reference Citation Analysis (0)] |