INTRODUCTION
Dendritic cells (DCs) play a key regulatory role in antitumor immunity, especially in its immune accessory role via MHC-I molecules[1-5]. We have recently reported that DCs were able to enhance the killing activity of Lymphokine and PHA activated killer (LPAK) cells in vitro[6-8]. In the present study, we evaluated the effects of GM-CSF and TNF upon antitumor activities of freshly isolated dendritic Cells in human peripheral blood (DC-0) and those cells cultivated for 36 h in vitro (DC-36). To perform such an evaluation, we compared killing effects of LPAK cells with addtional DC-0 or DC-36 on hepatoma cell line (BEL-7402) under regulation of GM-CSF or TNF. This study provided some basic data for further antitumor research.
MATERIALS AND METHODS
Tumor cell line
Human hepatoma cell line BEL-7402 was purchased from experimental center of Sun Yat-Sen University of Medical Sciences.
Isolation of DCs
According to our previous method[9], peripheral blood mononuclear cells (PBMNC) from healthy volunteers were prepared by using Ficoll-Hypaque (ρ = 1077 g/L) centrifugation method. Interface cells were collected and washed three times to remove platelets. Discontinuous Percoll density gradient centrifugation (Percoll: Pharmacia, Sweden) was employed, and then interface cells between 35% and 50% were collected which were called as preliminary enrichment of DCs and divided into two shares. One share (DC-0) was panned immediately; other one (DC-36) further cultured in PRMI-1640 with 100 mL/L inactivated fetal calf serum (100 mL/L FCS PRMI-1640) at 37 °C in a full humidified 50 mL/L CO2 atmosphere for 36 h, and panned. The non-adherent fractions of two shares as DC-0 and DC-36 were washed and collected for the experiments.
Preparation of LPAK cells[
9]
The PBMNCs were prepared in the same procedure as above, cultured 2 × 109/L- population with the final concentration of rhIL-21000 ku/L and PHA 20 mg/L in 100 mL/L FCS PRMI-1640 at 37 °C in a full humidified 50 mL/L CO2 atmosphere for 7 d. Half volume of the solution was replaced by fresh culture medium at the fourth day.
Anti-tumor experiment
The anti-tumor experiments were divided into two groups and each contained five experimental subgroups. Two ratios of effect (LPAK) to target (BEL-7402) (5:1 and 10:1) were used in all groups. ① DC-0 group: d group: BEL-7402 (8 × 107/L) + LPAK + DC-0 (8 × 106/L); g1 group: d group + GM-CSF (500 ku/L); g2 group: d group + GM-CSF (100 ku/L); t1 group: d group + TNF (5000 ku/L); t2 group: d group + TNF (500 ku/L); ② DC-36 group: each experimental group was the same as that in DC-0 group except DC-36 in place of DC-0 in the same concentration. These experimental groups were called D group, G1 group, G2 group, T1 group and T2 group respectively. In addition, L groups as the corresponding control groups, BEL-7402 + LPAK, experimental control group only consisted of BEL-7402, its population was 8 × 107/L. Culture medium control group only contained 100 mL/L FCS-PRMI-1640 with the supernatant of LPAK cells at a concentration of 50 μL/culture well. All of these groups were cultured in 96-well-culture plates and each group had 3 wells at 37 °C in a full humidified 50 mL/L CO2 atmosphere for 48 h. Cytotoxity assay was detected by using neural red uptake method.
Cytotoxicity assay (neural red uptake method)[
10]
0.1 mL 0.3 mL/L neural red solution was added in each well for another 1 h of culture. Following three washings with phosphate-buffered saline (PBS), 0.1 mL HCl-ethanol solution was added in each well. The absorption value (A value) of each well was immediately read by BIO-RAD 3550-UV type automatic ELISA reader at 570 nm wavelength. The formula of cytotoxicity is as follows: [1 - (Experimental group A - medium control group)/(Control group A - medium control group A)] × 100%. The experimental results were analyzed through analysis of variance by using GB-STAT statistic software. The experiment repeated four times at the same condition.
RESULTS
Influence of DC-0 and DC-36 on cytotoxity activity of LPAK cells
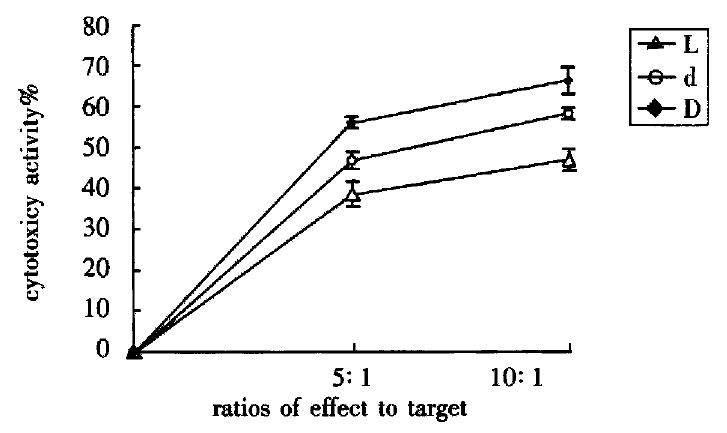
The cytotoxic activity of L group, d group and D group was enhanced when their ratios of effect to target increased (P < 0.01). Their cytotoxic activity were D group > d group > L group (P < 0.01) respectively while in the same ratio of effect to target (Figure 1).
Figure 1 Influence of DC-0 and DC-36 on LPAK cells in killing BEL-7402 cells in vitro.
Influence of GM-CSF on DC-0 and DC-36 in helping LPAK cells killing effect
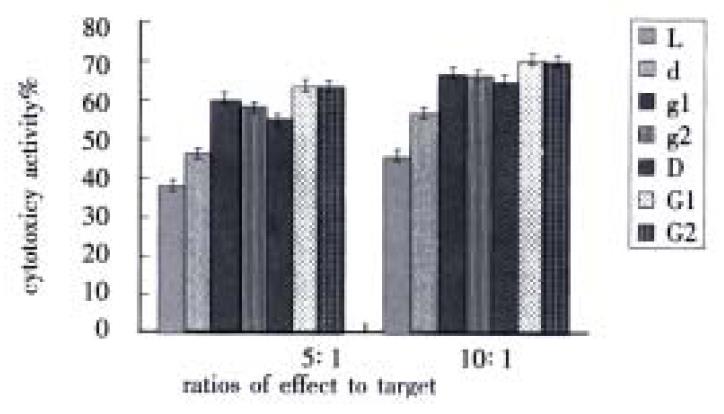
When there were two ratios of effect to target, cytotoxic activity of g1 group and g2 group were obviously higher than d group (P < 0.01), meantime, cytotoxic activity of G1 group and G2 group were greatly higher than D group (P < 0.01). However, the difference between g1 group and g2 group was not distinct (P > 0.05), also there were no difference between G1 group and G2 group (P > 0.05). But there were obviously different between g1 group and G1 group (P < 0.01), at the same time, the difference between g2 group and G2 group were distinct (P < 0.01) (Figure 2).
Figure 2 Influence of GM-CSF on DC-0 and DC-36 in helping LPAK cells killing activity in vitro.
Influence of TNF on DC-0 and DC-36 in helping LPAK cells killing effect
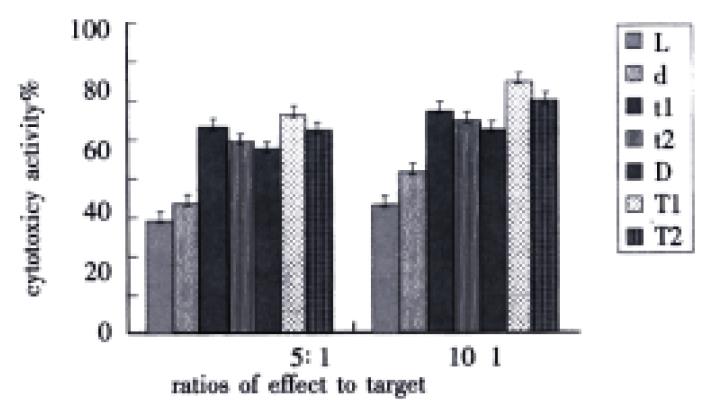
While there were two ratios of effect to target, cytotoxic activity of t1 group or t2 group was evidently higher than that of d group (P < 0.01), meantime, cytotoxic activity of T1 group or T2 group was markedly higher than that of D group (P < 0.01). However, the differences between t1 and t2 group, and between T1 and T2 group were distinct (P < 0.01). Furthermore, there were difference between t1 and T1 group (P < 0.01), at the same time, between t2 and T2 group (P < 0.01) (Figure 3).
Figure 3 Influence of TNF on DC-0 and DC-36 in helping LPAK cells killing activity in vitro.
DISCUSSION
In recent years, it is considered that mature DCs in human peripheral blood have high stimulating function, which efficiently presents tumor-peptide epitopes leading to induce cytotoxic T lymphocytes (CTL) to produce stronger specific antitumor immune response[11-14]. LPAK cells after 7-d induction chiefly express similar phenotype with the CD16-, CD8+, CD3+ CTL subtype[15-18]. In our experiments, cytotoxic activity in D group was obviously higher than in d group, which demonstrated that the proportion of mature DCs in DC-36 group was higher than those in DC-0 group and DC-36 group could stimulate LPAK cells to exert stronger antitumor immune response. This effect suggested that a lot of precursor cells of DCs and immature DCs in freshly isolated DCs could differentiate into mature DCs after 36 h cultivation, which coincided with Young’s opinion[19]. In human peripheral blood, however, not all the precursor cells and immature DCs are able to automatically differentiate into mature DCs in vitro. It is GM-CSF that promotes the differentiation, maturation and activation of DCs. GM-CSF can not only initiate and promote development of DCs from MHC II-, MHC II+ precursors and immature DCs, but also upregulated CD86- expression on DCs, which make DCs to have activating and controlling antitumor immune function[20-24]. Cytotoxic activity difference between G2 (g2) group and G1 (g1) group were not distinct, this finding illustrated that maturation and activation of DCs did not result from one factor but from combination of multiple factors. In addition, the different time required in different developing stage of DCs populations must be considered. Although developing time in DC-36 group was longer than that in DC-0 group, not all DCs in DC-36 group differentiated into mature DCs. Therefore, increasing GM-CSF concentration alone was senseless. This phenomenon may be helpful in further study of antitumor immunity and clinical research.
In the case of TNF addition, cytotoxic activity was increased greatly, this finding attributed to three roles of TNF: 1, TNF is able to serve as the first signal, which affects DCs development in their early stage or whole stage, leading to upregulate the GM-CSF receptor level of DCs[21]. The supernatant of LPAK cells with minor quantity of cell growth factors such as TNF was added into culture medium to afford synergetic effect with GM-CSF; 2, TNF upregulates expression of CD80, CD83, CD86 and MHC-II in a short period[25,26]. Because these molecules are crucial for efficient antigen presenting, they promote the differentiation, development and activation of DCs. 3, TNF itself can kill tumor cells directly[27-32]. Compared with two t groups, cytotoxic activity of T1 and tª-1 groups were higher than that of T2 and t2 groups, which showed that in DC-36 groups there was plenty of time for immature DCs to evolve into mature DCs after the addition of TNF. Furthermore, T1 (t1) group had higher cytotoxic activity than T2 (t2) group, which was further increased when TNF dosage was raised. This phenomenon may attribute to antitumor effect of TNF itself and the synergetic effect between LPAK cells and TNF.
In conclusion, as compared with uncultured DC-0, cultured DC-36 from freshly isolated DCs had greater cooperative effect with GM-CSF or TNF. Moreover, they enable DCs to fulfill stronger antitumor effect.