Published online Oct 15, 2000. doi: 10.3748/wjg.v6.i5.709
Revised: May 20, 2000
Accepted: June 2, 2000
Published online: October 15, 2000
AIM: To clone expressed genes associated with repair of irradiation-damaged mice intestinal gland cells treated by small intestinal RNA, and to explore the molecular mechanism of exogenous nucleic acids improving repair of intestinal crypt.
METHODS: The animal mode of test group and control group was established, forty-five mice being irradiated by γ ray were treated with small intestinal RNA as test group, forty mice being irradiated by γ ray were treated with physiological saline as control group, five mice without irradiation were used as normal control, their jejunal specimens were collected respectively at 6 h, 12 h, 24 h, 4 d and 8 d after irradiation. Then by using LD-PCR based on subtractive hybridization, these gene fragments differentially expressed between test group and control group were obtained, and then were cloned into T vectors as well as being sequenced. Obtained sequences were screened against. GeneBank, if being new sequences, they were submitted to GeneBank.
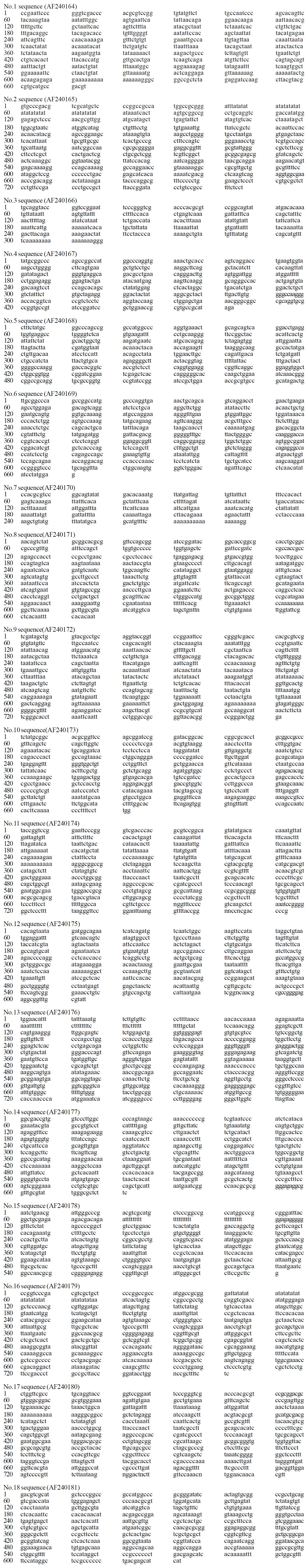
RESULTS: Ninety clones were associated with repair of irradiation-damaged intestinal gland cells treated by intestinal RNA. These clones from test group of 6 h, 12 h, 24 h, 4 d and 8 d were respectively 18, 22, 25, 13, 12. By screening against GeneBank, 18 of which were new sequences, the others were dramatically similar to the known sequences, mainly similar to hsp, Nmi, Dutt1, alkaline phosphatase, homeobox, anti-CEA ScFv antibody, arginine/serine kinase and BMP-4, repA. Eighteen gene fragments were new sequences, their accept numbers in GeneBank were respectively AF240164-AF240181.
CONCLUSION: Ninety clones were obtained to be associated with repair of irradiation-damaged mice intestinal gland cells treated by small intestinal RNA, which may be related to abnormal expression of genes and matched proteins of hsp, Nmi, Dutt1, Na, K-ATPase, alkalineph-osphatase, glkA, single stranded replicative centromeric gene as well as 18 new sequences.
- Citation: Cui DX, Zeng GY, Wang F, Xu JR, Ren DQ, Guo YH, Tian FR, Yan XJ, Hou Y, Su CZ. Mechanism of exogenous nucleic acids and their precursors improving the repair of intestinal epithelium after γ-irradiation in mice. World J Gastroenterol 2000; 6(5): 709-717
- URL: https://www.wjgnet.com/1007-9327/full/v6/i5/709.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i5.709
After exposure to large dose ionizing radiations, the larger intestinal gland cell lesion is the main cause of death in humans and animals. How to enhance intestinal gland cell survival rates has become a problem to be solved. In order to increase the mouse crypt survival after irradiation, we have done a series of experiments, and finally confirmed that when a portion of the crypts was devastated by irradiation, a compensatory recovery of the intestinal epithelium by remaining crypts occurred involving three consecutive periods such as the rapid cell proliferation of the viable crypts, the fission of the proliferative crypts and the increase of crypt numbers[1]. The nucleic acid fragment containing several hundred base pairs (bp), or even any one of the nucleic acid precursors (mononucleotides, nucleosides, and bases) can enhance the crypt survival rate by 25% or so, further confirming that the effectiveness of exogenous nucleic acids depends not upon the action exerted by their highly polymerized state, but upon their various enzymatic degradation products[2]. Our experimental results suggest that the nucleic acids (DNA, RNA) and their precursors may be used as one of the effective measures for the treatment of intestinal radiation syndrome that may occur in the war time as well as in the peaceful use of atomic energy[3-5]. However, the molecular mechanism how nuclear acids and precursors improve the repairing of irradiation-damaged intestinal gland cells is unclear. In order to clarify this molecular mechanism, we used long distance-PCR based on subtractive hybridization, isolated and cloned these genes associated with repairs of irradiation-damaged mice intestinal gland cells treated by intestinal RNA. Our studies lay foundation for further clarifying molecular mechanism of repair of radiation-damaged crypt.
PolyATtract® system 1000 kit from Promega was used for extraction of mRNA, SMART PCR cDNA synthesis kit (Clontech) for transcription of mRNA, Wizard® plus Minipreps DNA purification for purification of PCR production, Advantage2PCR kit (Clontech) for LD-PCR, and PE-5700 quantiative PCR cycler used for thermal cycle. PGEM-T easy vector system was purchased from Promega, γ-32-pdATP from Beijing Fu Rei Compancy, and the other reagents were from Beijing Yuan Ping compancy.
Ninety BALB/c male mice with body weight of 18 g-22 g and 10-12 weeks old were randomly assigned into two groups, i.e, test group and control group. They were injected with 5 g·L-1 barbiturate sodium 40 mg·kg-1, put in organism radiation box, and then were irradiated on mice abdominal region by using 60Co γ ray at the reagent rate of 149.47-151.13 cGy·min-1, and finally reached total reagent of 1150 cGy. Small intestinal RNA was diluted into 100 mg·mL-1. Two hours after mice being irradiated, each mouse in test group was injected 0.4 mL RNA liquid, and each mouse in control group was injected 0.4 mL physiological saline by using local intestinal cavity expanding injection method[6]. After that, the mice were raised according to conventional method. These mice were killed respectively at 6 h, 12 h, 24 h, 4 d and 8 d after irradiation, and jejunal tissues were quickly taken out, washed by physiological saline, and then were kept in liquid nitrogen. In order to avoid single difference, these samples were mixed together under identical condition.
Extraction of mRNA was done according to manual of PolyATtract system 1000, and quantificated. mRNA transcription was done according to manual from SMART PCR cDNA synthesis kit. Subtractive hybridization between test group and control group was done as follows: take out 0.1 µg mRNA from the control group, add Biotinylated oligo (dT) probe (50 µmol·L-1), 70 °C 5 min, then add into the first strand cDNA, and hybridize for 24 h at 42 °C, finally add Streptavidin magnesphere particles and mixed, magnetic steel was used to attract production of two stranded hybrids and get rid of them, and then repeat the processing twice. Take out the upper liquid, and add double volume absolute alcohol to the upper liquid to precipitate cDNAs, finally dissolve them in 10 µL Nuclease-free water. Take 10 µL of the first strand cDNA as template, sequentially add PCR-grade water 74 µL, 10 × advantage 2 PCR buffer 10 µL, 50 × dNTP mix-10 µL, 50 × advantage 2 polymerase mix 1 µL, PCR primer Mix 2 µL, 50 mmol·L-1 MgCl2 3 µL, mixed well, centrifuged for several seconds, using two steps such as 95 °C,1 min, 95 °C,15 s, 68 °C, 6 min, 30 cycles, in the end elongated at 68 °C for 6 min, take out 10 µL production to run 12 g·L-1 agarose gel electrophoresis.
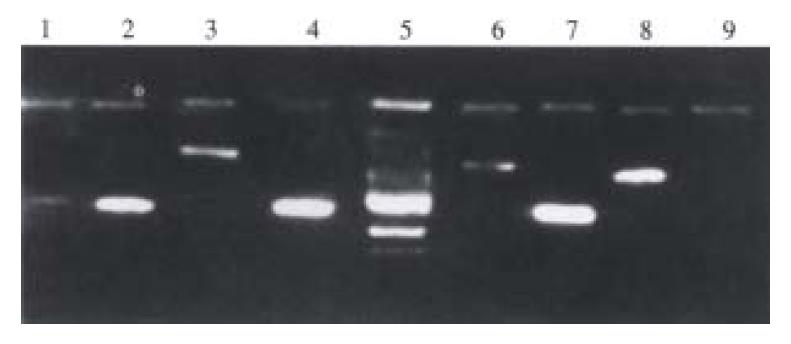
PCR products were purified using Wizard plus Minipreps DNA purification system, and dissolved in 10 µL water. According to the manual, PCR products were cloned into PGEM-T easy vector, transferred into JM109, positive clone picked up, cultured overnight, plasmid extracted out, and identified by cutting with BstZ1. Products cut by BstZ1 were labelled γ-32pdATP at terminal, and used as probe, and hybridized with mRNA of test group and control group[7].
These obtained clones were positively sequenced by using Model 377 sequencing instrument. Obtained sequences were screened against GeneBank, if being new sequences, these sequences were submitted to GeneBank[8].
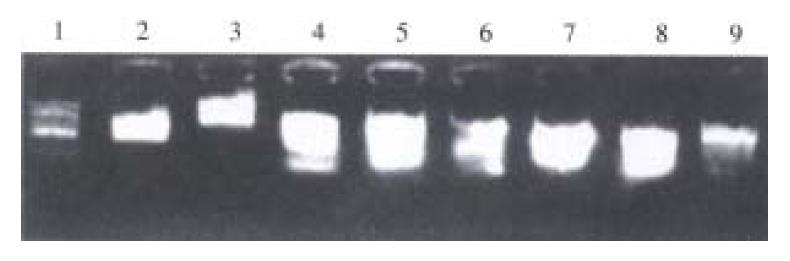
In test group of 6 h, 12 h, 24 h, 4 d and 8 d, products after subtractive hybridization were successfully amplified by LD-PCR, obtained bands centered on 1-1.5 kb or so (Figure 1).
These clones were sequenced according to the results of sequencing and searching against GeneBank, eighteen were new sequences, eighty-two were dramatically similar to the known sequences.
In test group of 6 h, similar sequences mainly were as follows: mRNA for heat shock protein, Nmi mRNA, Dutt1 protein, mRNA for Na, K-ATPase gamma subunit, mRNA for surface glycoprotein, Zinc finger type transcript factor, porcine growth hormone-releasing hormone gene, monocyte/macrophage Igrelated gene, telomerase-associated protein, HOX1b protein, arginine/ serine kinase.
In test group of 12 h, similar sequences were: Alkaline phosphatase mRNA, alkaline phosphatase 2, glkA gene, single stranded replicative centromeric gene, DMBT1, tRNA-Met gene, homeobox protein, thyroxine-binding globulin gene, alpha-a-plasmin inhibitor gene.
In test group of 24 h, similar sequences were: anti-CEA ScFv antibody, anti-DNA heavy chain, mRNA for Ig kappa chain, anti BONT/A Hc ScFv antibody, mRNA for collagenase, AE0199 immunoglobulin heavy chain, Mouse Ig gamma-chain, Ig rearranged gamma-chain mRNA, anti-c-myc antibody, anti-CD30 moab ki4 ScFv, anti-BSA antibody, D1 heavy chain, epidermal growth factor, anti-NP antibody IgH, mRNA for arginine/serine kinase.
In test group of 4 d, simliar sequences were: Dual specificity phosphatase, family mRNA telomerase-associated protein, anti-human erbB-2, tazarotene-induced gene2, betaine-GABA transporter gene, copy complex subunit 7a, mRNA for stress-activated protein, FK506 binding protein, calium/calmodulin dependent gene, PEST phosphatase interactin gene, haptoglobin mRNA, acyl-ACP desaturase, mRNA for sodium channel, peroxidase, BMP-4 gene, bone morphogenetic protein.
In test group of 8 d, similar sequences were: Ig variable region, DNA for mouse Ig, DNA for flexible peptide, tsr glkA, proteinase-3 neutroactin, EWS gene, repA protein.
Eighteen new sequences Figure 4
After exposing to large dose ionizing radiation, intestinal crypt radiation death occurs, and no effective therapeutic measures are available to combat it. Data showed that the devastation or death of the crypt after irradiation is the crucial factor responsible for the pathogenesis. We have performed a series of experiments intending to increase the crypt survival after-irradiation in mice and confirmed that the nucleic acids (DNA, RNA) and their precursors may be used as one of the measures for the treatment of intestinal radiation syndrome that may occur in the war as well as in the peaceful uses of atomic energy[1-5]. However, the concrete molecular and cellular mechanisms are unknown.
Human genome group comprised 100 thousand of genes, which are selectively expressed, and determined the whole life course of organism, alteration of gene expressed levels is positioned at the centrel of controlling biological adjust mechanism[8]. Therefore, we think, after irradiation, between test group treated by RNA and the control group treated by physiological saline must exist differently expressed genes, which indicate that those genes were closely associated with intestinal crypt damage and repair. To isolate and clone these genes may not only be helpful to clarify the molecular mechanism of nuclear acids treatment, but also provide important basic theory for gene therapy of irradiation damage.
In the study, using BALB/c mice as studying target, we obtained 90 of genes a ssociated with repair of irradiation damaged intestinal gland cells. Data confirmed that hsp was increased at mRNA level after chronic radiation, PARP, serine protease-like gene, p53, bcl-2, bax, argainase I, ihsr PB7, Cdx1, NPT, PCNA, D1b-1, c-Ha-ras, c-myc, c-fos and so on were also increased at mRNA levels, which were correlated closely with drug treatment of irradiation damaged intestinal cells[9-25]. In our experiment, such as Nmi mRNA, Dutt1 protein, mRNA for Na, K-ATPase gamma subunit, mRNA for surface glycoprotein, Zinc finger type transcript factor, porcine growth hormone-releasing hormone gene, monocyte/macrophage Ig-related gene, telomerase-associated protein, HOX1b protein, arginine/serine kinase, alkaline phosphatase mRNA, alkaline phosphatase 2, glkA gene et al were also closely correlated with repair of irradiation damaged intestinal crypt, what especially interesting was that RSG5 and ODC were identical to obtained sequences, data showed that RSG5, and ODC were overexpressed in irradiation-damaged intestinal crypt, and played an essential and positive role during DNA damage recovery and survival[26,27], our results also fully supported the conclusion. Although their concrete mechanism is not clarified, they may increase protein products by means of increased transcript levels to improve repair of irradiation-damaged intestinal crypt, and to suppress apoptosis of crypt cells[32].
Langberg et al[28] confirmed that immunolo-gical factors participated in the course of repair of irradiation damaged intestinal crypt such as IL-1, TGF-beta1, PDGF-AA, c-EGFR, EGF, TGF-beta-3. In our experiment, anti-CEA ScFv antibody gene, anti-DNA heavy chain, mRNA for Ig kappa chain, anti-BONT/A Hc ScFv antibody gene, mRNA for ScFv collagenase, AE0199 immunoglobulin heavy chain, mouse Ig gamma-chain, Ig rearranged gamma-chain mRNA, anti-c-myc antibody gene, anti-CD30 mAb ki-4 ScFv, anti-BSA antibody gene, D1 heavy chain, epidermal growth factor, anti-NP antibody IgH, mouse Ig gammachain and haptoglobin were likely to be correlated closely with repair of irradiation damaged intestinal crypt. What is especially interesting is several gene fragments were partly identical to sequences of ScFv genes, this point was not able to be expressed clearly. Our results support that immunological factors exert effect on the course of repair of irradiation damaged intestinal crypt[29-45].
In our experiment, eighteen novel sequences were obtained, their concrete functions are still unclear. But we believe that these genes are closely associated with irradiation treatment, only if we clarify the function of these genes, and according to the changes of these genes, to design a controlling measure, we are likely to decrease irradiation damage, and also provide new thoughts for tumor radiation treatment[46-64].
In summary, our results primarily demonstrate that nuclear acids are capable of improving repair of irradiation damaged intestinal crypt, its action may be closely correlated with increased mRNA levels of some genes, also with immunological factors, but the concrete molecular mechanism such as signal transduction and suppression of apoptosis still needs further studies[65-89].
Edited by You DY and Ma JY
| 1. | Cui DX, Yan XJ, Su CZ. Differentially expressed genes in GC7901 or GES 1 were isolated by optimized differential display RT PCR and their primary clinical significance. Disi Junyi Daxue Xuebao. 1998;19:601-605. |
| 3. | Zeng GY, Liu AP, Zhou YK. Effect of exogenous nucleic acids and their precursors on the intestinal crypt survival in mice after abdominal γ - irradiation. China-Janan Medical Conference Beijing. 1992;5:968. |
| 4. | Zhou YK, Zeng GY, Liu AP. Effect of intraluminal administration of intes-tinal crypt cells on the crypt of lieberkuhn in irradiated mice. Internatinal Confere nce on Biological Effects of Large Dose lonizing and Nonionzing radiation Hangzhou, China, 1988: 125. . |
| 5. | Cui DX, Zen GY, Yan XJ, Ren DQ, Wang F, Zhao T, Tian FR, Su CZ. Differentially expressed genes related with injury of human intestinal epithe-lium cell by γ-ray. J Radiat Res Radiat Proces. 2000;18:73-76. |
| 6. | Cui DX, Zen GY, Yan XJ, Ren DQ, Wang F, Zhao T, Tian FR, Su CZ. Differen tially gene expression of mice radiation injury repair in RNA treated intestinal cells. Disi Junyi Daxue Xuebao. 2000;21:256-257. |
| 7. | Yong ML. World Wide Web and computer software in molecular biology. A collection of Papers 97 Beijing International Conference of Medical and Biology High tech 1997; 10: 9-15. . |
| 8. | Cui DX, Yan XJ, Su CZ. Advance of cloning technique of differentially expressed genes. Chem Life. 1999;19:232-235. |
| 9. | Melkonyan HS, Ushakova TE, Umansky SR. Hsp70 gene expression in mouse lung cells upon chronic gamma-irradiation. Int J Radiat Biol. 1995;68:277-280. [PubMed] |
| 10. | Subramanian V, Meyer B, Evans GS. The murine Cdx1 gene product localises to the proliferative compartment in the developing and regenerating intestinal epithelium. Differentiation. 1998;64:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | St Clair WH, Billings PC, Kennedy AR. The effects of the Bowman-Birk protease inhibitor on c-myc expression and cell proliferation in the unirradiated and irradiated mouse colon. Cancer Lett. 1990;52:145-152. [PubMed] |
| 12. | Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54:614-617. [PubMed] |
| 13. | St Clair WH, St Clair DK. Effect of the Bowman-Birk protease inhibitor on the expression of oncogenes in the irradiated rat colon. Cancer Res. 1991;51:4539-4543. [PubMed] |
| 14. | Erdman SH, Ignatenko NA, Powell MB, Blohm-Mangone KA, Holubec H, Guillén-Rodriguez JM, Gerner EW. APC-dependent changes in expression of genes influencing polyamine metabolism, and consequences for gastrointestinal carcinogenesis, in the Min mouse. Carcinogenesis. 1999;20:1709-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Hauer-Jensen M, Richter KK, Wang J, Abe E, Sung CC, Hardin JW. Changes in transforming growth factor beta1 gene expression and immunoreactivity levels during development of chronic radiation enteropathy. Radiat Res. 1998;150:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Tishkoff DX, Amin NS, Viars CS, Arden KC, Kolodner RD. Identification of a human gene encoding a homologue of Saccharomyces cerevisiae EXO1, an exonuclease implicated in mismatch repair and recombination. Cancer Res. 1998;58:5027-5031. [PubMed] |
| 17. | Tucker JD, Sorensen KJ, Chu CS, Nelson DO, Ramsey MJ, Urlando C, Heddle JA. The accumulation of chromosome aberrations and Dlb-1 mutations in mice with highly fractionated exposure to gamma radiation. Mutat Res. 1998;400:321-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Møller P, Wallin H, Dybdahl M, Frentz G, Nexø BA. Psoriasis patients with basal cell carcinoma have more repair-mediated DNA strand-breaks after UVC damage in lymphocytes than psoriasis patients without basal cell carcinoma. Cancer Lett. 2000;151:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Rodrigo G, Roumagnac S, Wold MS, Salles B, Calsou P. DNA replication but not nucleotide excision repair is required for UVC-induced replication protein A phosphorylation in mammalian cells. Mol Cell Biol. 2000;20:2696-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Ross GM. Induction of cell death by radiotherapy. Endocr Relat Cancer. 1999;6:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Parshad R, Ning Y, Sanford KK. Suppression of X-ray-induced chromatid breaks in human tumor cells by introduction of normal chromosome 4. Cancer Genet Cytogenet. 2000;118:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Chao KS, Hsu JS, Xu J, Ezekiel UR, Eves E, Rosner M, Hsu CY. Differential effect of cycloheximide on neuronal and glioma cells treated with chemotherapy and radiation. J Neurooncol. 1999;45:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Koufen P, Stark G. Free radical induced inactivation of creatine kinase: sites of interaction, protection, and recovery. Biochim Biophys Acta. 2000;1501:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Lee JS, Collins KM, Brown AL, Lee CH, Chung JH. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 400] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Dittmann KH, Dikomey E, Mayer C, Rodemann HP. The Bowman-Birk protease inhibitor enhances clonogenic cell survival of ionizing radiation-treated nucleotide excision repair-competent cells but not of xeroderma pigmentosum cells. Int J Radiat Biol. 2000;76:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Sikpi MO, Wang Y. Ionizing radiation enhances double-strand-break repair in rapamycin-treated ataxia telangiectasia lymphoblasts. Int J Radiat Biol. 2000;76:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Sachs RK, Rogoff A, Chen AM, Simpson PJ, Savage JR, Hahnfeldt P, Hlatky LR. Underprediction of visibly complex chromosome aberrations by a recombinational-repair ('one-hit') model. Int J Radiat Biol. 2000;76:129-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Langberg CW, Hauer-Jensen M, Sung CC, Kane CJ. Expression of fibrogenic cytokines in rat small intestine after fractionated irradiation. Radiother Oncol. 1994;32:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | de Gruijl FR. Skin cancer and solar UV radiation. Eur J Cancer. 1999;35:2003-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 328] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 30. | Carlomagno F, Burnet NG, Turesson I, Nyman J, Peacock JH, Dunning AM, Ponder BA, Jackson SP. Comparison of DNA repair protein expression and activities between human fibroblast cell lines with different radiosensitivities. Int J Cancer. 2000;85:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | May A, Bohr VA. Gene-specific repair of gamma-ray-induced DNA strand breaks in colon cancer cells: no coupling to transcription and no removal from the mitochondrial genome. Biochem Biophys Res Commun. 2000;269:433-437. [PubMed] |
| 32. | Ashush H, Rozenszajn LA, Blass M, Barda-Saad M, Azimov D, Radnay J, Zipori D, Rosenschein U. Apoptosis induction of human myeloid leukemic cells by ultrasound exposure. Cancer Res. 2000;60:1014-1020. [PubMed] |
| 33. | Bishay K, Ory K, Lebeau J, Levalois C, Olivier MF, Chevillard S. DNA damage-related gene expression as biomarkers to assess cellular response after gamma irradiation of a human lymphoblastoid cell line. Oncogene. 2000;19:916-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Minami K, Matsuzaki S, Hayashi N, Mokarim A, Ito M, Sekine I. Immunohistochemical study of p53 overexpression in radiation-induced colon cancers. J Radiat Res. 1998;39:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Gotoh T, Araki M, Mori M. Chromosomal localization of the human arginase II gene and tissue distribution of its mRNA. Biochem Biophys Res Commun. 1997;233:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Komarova EA, Chernov MV, Franks R, Wang K, Armin G, Zelnick CR, Chin DM, Bacus SS, Stark GR, Gudkov AV. Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J. 1997;16:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 228] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Ruifrok AC, Mason KA, Lozano G, Thames HD. Spatial and temporal patter ns of expression of epidermal growth factor, transforming growth factor alpha and transforming growth factor beta1 3 and their receptors in mouse jejunum after radiation treatment. Radiat-Res. 1997;147:1-12. |
| 38. | Arai T, Kida Y, Harmon BV, Gobé GC. Expression and localization of clusterin mRNA in the small and large intestine of the irradiated rat: its relationship with apoptosis. Int J Radiat Biol. 1996;69:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Blazek J. Kinetics and morphology of immune reactive cells in the spleen as observed using the immune adherence method. IV. Primary-esponse as influenced by total body irradiation in various intervals following administrati on of antigen. Cesk Patol. 1976;12:89-103. |
| 40. | Blazek J. Kinetics and morphology of immune reaction cells in the spleen studied using the immuno-cyto-adherence reaction. V. Secondary response and its modification by whole body irradiation at different intervals following administration of antigen. Cesk Patol. 1976;12:209-220. |
| 41. | Schwarze G, Dietz R. [Animal experimental studies on the increase in the humoral immune response caused by whole body irradiation]. Strahlenther Onkol. 1988;164:746-751. [PubMed] |
| 42. | Blazek J. [Immunopathological aspects of nucleolar activation. 1. Changes of nucleolar activation during the course of primary immune response]. Cesk Patol. 1977;13:52-63. [PubMed] |
| 43. | Blazek J. Cell kinetics and cell morphology of the splenic immunity reac-tion as followed by the immunocytoadherence method. II. Secondary response. Cesk Patol. 1974;10:37-42. |
| 44. | Lubbe FH, Zaalberg OB. Enhancing effect of radioresistant spleen cells on the primary immune response against sheep RBC by mouse spleen cells in vitro. Adv Exp Med Biol. 1976;66:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 45. | Masihi KN, Werner H. Rosette-forming cells during immune response to Toxoplasma gondii in mice. Infect Immun. 1976;13:1678-1683. [PubMed] |
| 46. | Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, Takei Y, Nakamura Y. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 47. | Gevorkian SK, Dergachev VI, Iarilin AA, Filatov PP. [Effect of the spleen extracts on primary immune response in mice]. Biull Eksp Biol Med. 1976;82:1228-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Marbrook J. Primary immune response in cultures of spleen cells. Lancet. 1967;2:1279-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 300] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Maksimova GF, Babichev VA, Uteshev BS. [Autoradiographic study of the spleen at an early stage of the primary immune response]. Dokl Akad Nauk SSSR. 1972;207:1467-1470. [PubMed] |
| 50. | Romashevskaia EI, Khasman EL. [Role of different fractions of adhering splenocytes in inducing antibody formation in irradiated mice]. Zh Mikrobiol Epidemiol Immunobiol. 1980;8:91-94. [PubMed] |
| 51. | Watanabe S, Kajiwara H, Minowada J, Yamamura Y. X-ray enhancement of splenic rosette-forming cells in nonimmune mice. Eur J Immunol. 1976;5:486-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Marchal G. [Increased catabolism of antigen and enhanced recruitment of antigen-sensitive cells by activation of macrophages with a bacterial phospholipid extract (EBP) (author's transl)]. Ann Immunol (Paris). 1979;130:901-917. [PubMed] |
| 53. | Rossi G, Zaalberg OB. The induction of a primary immune response in spleen cells cultured in diffusion chambers. Proc K Ned Akad Wet C. 1971;74:92-104. [PubMed] |
| 54. | Zhang XC, Gao RF, Li BQ, Ma LS, Mei LX, Wu YZ, Liu FQ, Liao ZL. Clinical and experimental study of therapeutic effect of Weixibaonizhuan pills on gastric precancerous lesions. World J Gastroenterol. 1998;4:24-27. [PubMed] |
| 55. | Chen DZ, Wei MX. Preliminary study on the pathological model of Piyinxu in rats. China Natl J New Gastroenterol. 1997;3:120. |
| 56. | Mi JQ, Yang SQ, Shen MC. The expression of cerbB 2 proto oncogene product in gastric carcinoma and precancerous lesions. China Natl J New Gastroenterol. 1997;3:122. |
| 57. | Ji XL, Cheng YQ, Wang SQ. Gastroendoscopic biopsy diagnosis of mu-cosa associated lymphoid tissue lyphoma. China Natl J New Gastroenterol. 1995;1:30-32. |
| 58. | Zhuang XQ, Yuan SZ, Wang XH, Lai RQ, Luo ZQ. Oncoprotein expres-sion and inhibition of apoptosis during colorectal tumorigenesis. China Natl J New Gastroenterol. 1996;2:3-5. |
| 59. | Li ZX, Liu PY, Xu WX, Cong B, Ma ZX, Li Y. p53 gene mutations in primary gastric cancer. China Natl J New Gastroenterol. 1996;2:41-43. |
| 60. | Yin GY, He XF, Yin YF. Clinical and experimental study on gastric mucosal pathology, DNA, cAMP, and trace elements of pixu patients. China Natl J New Gastroenterol. 1996;2:44-50. |
| 61. | Wang LD, Zhou Q, Gao SS, Li YX, Yang WC. Measurements of cell proliferation in esophageal and gastric cardia epithelia of subjects in a high incidence area for esophageal cancer. China Natl J New Gastroenterol. 1996;2:82-85. |
| 62. | Chen XM, Xu RL, Ma XH, Zhou YC, Han DW. Mucosal permeability to lipopolysaccharides in the colon in chronic alcoholic rats. China Natl J New Gastroenterol. 1996;2:125-127. |
| 63. | Tao HQ, Qin LF, Lin YZ, Wang RN. Expression of vascular endothelial growth factor and its prognostic significance in gastric carcinoma. China Natl J New Gastroenterol. 1996;2:128-130. |
| 64. | Yu JY, D' Adda T. Quantitative ultrastructure analysis of neuroendocrine cells of gastric mucosa in normal and pathological conditions. China Natl J New Gastroenterol. 1996;2:155-157. |
| 65. | Chen GZ, Fu D. Effects of Jiawei Sijunzi Tang Decoction on migrating m yoelectric complex in 8.0Gy irradiated rats. China Natl J New Gastroenterol. 1996;2:197-199. |
| 66. | Ruan CP, Wang YH, Wang LG, Wang YX. Changes of neurotensin and endotox in in rats with intestinal ischemia. China Natl J New Gastroenterol. 1996;2:200-202. |
| 67. | Zhu SL, Xu GS, Chen QZ, Wang ZJ, Jiao J. The effects of electroneedling at point Zusanli on stress gastric ulcer: The changes of nitric oxide and cate cholamine in rats. China Natl J New Gastroenterol. 1996;2:203-205. |
| 68. | Peng DF, Lin HM. Expression of P53 oncoprotein in benign and malignant lesions of large bowel. China Natl J New Gastroenterol. 1996;2:236-237. |
| 69. | Tang FC, Zhang YF, Xu YD, Zhong SQ, Wang XP, Wang YX. Scanning electron microscopic studies of lymphatic corrosion casts in the rabbit appendix. China Natl J New Gastroenterol. 1996;2:238-240. |
| 70. | Chen X, Noguchi K, Tanikawa K. [Uptake of Escherichia coli lipopolysaccharide and expression of tumor necrosis factor-alpha-mRNA by isolated rat intrahepatic bile duct epithelial cells]. Zhonghua Binglixue Zazhi. 1997;26:78-81. [PubMed] |
| 71. | Gu SQ, Liang YY, Fan LR, Li BY, Wang DS. Co regulative effects of the cAMP/PKA and DAG/PKC signal pathways on human gastric cancer cells during diffe rentiation induced by traditional Chinese medicines. China Natl J New Gastroenterol. 1997;3:50-53. |
| 72. | Zhang AL, Chen RX, Kang MF, Fan HL, Wang WL. Study on regulatory effect of acupuncture on rotation induced gastric dysrhythmia in rabbits. China Natl J New Gastroenterol. 1997;3:54-55. |
| 73. | Sun WB, Ma RL, Peng ZM, Li K, Duan HC, Han BL. Protective effect of vitamin Eon the age related alterations of Kupffer cell's energy metabolism. China Natl J New Gastroenterol. 1997;3:78-80. |
| 74. | Chen DC, Yang XY, Zhang XY, Chen XY. Protective effect of rhubarb on barrier of intestinal mucosa. China Natl J New Gastroenterol. 1997;3:81-83. |
| 75. | Jiang CP, Chen YQ, Zhu JW, Shen HX, Yu X. Immunohistochemical study of gastrin in colorectal carcinoma tissues and its adjacent mucosa. China Natl J New Gastroenterol. 1997;3:84-86. |
| 76. | Wang LD, Yang WC, Zhou Q, Xing Y, Jia YY, Zhao X. Changes of p53 and Waf1p21 and cell proliferation in esophageal carcinogenesis. China Natl J New Gastroenterol. 1997;3:87-89. |
| 77. | Zhang ZG, Wu JY, Fu XD, Gu DK, Fang F. P21 and CEA expression and AgN OR counts in DMH induced colon carcinoma in rats. China Natl J New Gastroenterol. 1997;3:163-165. |
| 78. | Zhang L, Zhang ML, Yan YQ, Liang DX. Basal level of epidermal growth factor in gastric juice of 86 healthy Chinese volunteers by radioimmunoassay. China Natl J New Gastroenterol. 1997;3:245. |
| 79. | Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421-425. [PubMed] |
| 80. | Yu GQ, Zhou Q, Ivan D, Gao SS, Zheng ZY, Zou JX, Li YX, Wang LD. Changes of p53 protein blood level in esophageal cancer patients and normal subjects from a high incidence area in Henan, China. World J Gastroenterol. 1998;4:365-366. [PubMed] |
| 81. | Chen XQ, Zhang WD, Song YG, Zhou DY. Induction of apoptosis of lymphocytes in rat mucosal immune system. World J Gastroenterol. 1998;4:19-23. [PubMed] |
| 82. | Zhang XC, Gao RF, Li BQ, Ma LS, Mei LX, Wu YZ, Liu FQ, Liao ZL. Clinical and experimental study of therapeutic effect of Weixibaonizhuan pills on gastric precancerous lesions. World J Gastroenterol. 1998;4:24-27. [PubMed] |
| 83. | Sun WB, Han BL, Peng ZM, Li K, Ji Q, Chen J, Wang HZ, Ma RL. Effect of aging on cytoskeleton system of Kupffer cell and its phagocytic capacity. World J Gastroenterol. 1998;4:77-79. [PubMed] |
| 84. | Yan JP, Jia JB, Ma XH, Wu XR, Zhao YC, Han DW. Immunohis-tochemical study on expression of epidermal growth factor receptor at hepa-tocyte nuclei in experimental rat liver cirrhosis. World J Gastroentero. 1998;4:143. |
| 85. | Xiao L, Zhou HY, Luo ZC, Liu J. Telomeric associations of chromosomes in patients with esophageal squamous cell carcinomas. World J Gastroenterol. 1998;4:231-233. [PubMed] |
| 86. | Göke M, Kanai M, Lynch-Devaney K, Podolsky DK. Rapid mitogen-activated protein kinase activation by transforming growth factor alpha in wounded rat intestinal epithelial cells. Gastroenterology. 1998;114:697-705. [PubMed] |
| 87. | Assy N, Gong Y, Zhang M, Minuk G. Appearance of an inhibitory cell nuclear antigen in rat and human serum during variable degrees of hepatic regenerative activity. World J Gastroenterol. 1999;5:103-106. [PubMed] |
| 88. | Assy N, Paizi M, Gaitini D, Baruch Y, Spira G. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol. 1999;5:296-300. [PubMed] |
| 89. | Zhan WH, Ma JP, Peng JS, Gao JS, Cai SR, Wang JP, Zheng ZQ, Wang L. Telomerase activity in gastric cancer and its clinical implications. World J Gastroenterol. 1999;5:316-319. [PubMed] |
| 90. | Zheng L, Gao ZQ, Wang SX. A chronic ulcerative colitis model in rats. World J Gastroenterol. 2000;6:150-152. [PubMed] |