Published online Oct 15, 2000. doi: 10.3748/wjg.v6.i5.688
Revised: May 9, 2000
Accepted: May 16, 2000
Published online: October 15, 2000
AIM: To study the effect of hepatocyte apoptosis and necrosis induced by TNF-α on the pathogenesis of acute severe hepatitis (ASH).
METHODS: The model of ASH was prepared in D-galactosamine (GalN) sensitized BALB/c mice by injection of either endotoxin (ET) or tumor necrosis factor-α (TNF-α). Morphological changes of apoptotic hepatocytes were studied by both light and electron microscope and in site end labeling method (ISEL). Molecular biological changes of DNA ladder were observed by electrophoresis of extract from liver tissues. Biochemical changes were measured by alanine aminotransferase (ALT), aspartic aminotransferase (AST) and TNF-α. The relation between apoptosis and necrosis was evaluated simultaneously.
RESULTS: The sequence of hepatocyte apoptosis, necrosis, and final death from ASH was observed both in GalN/ET and GalN/TNF-α group. Apoptosis was prominent at 3.5 h and 6 h after injection of inducer, while necrosis became dominant at 9 h after challenge. The appearance of apoptosis was earlier in GalN/TNF-α group than that in GalN/ET group. Pretreatment of mice with antiTNF IgG1 may completely prevent the liver injury induced by GalN/ET.
CONCLUSION: TNF-α can cause liver damage by inducing hepatic apoptosis and necrosis in mice with endotoxemia.
- Citation: Zang GQ, Zhou XQ, Yu H, Xie Q, Zhao GM, Wang B, Guo Q, Xiang YQ, Liao D. Effect of hepatocyte apoptosis induced by TNF-α on acute severe hepatitis in mouse models. World J Gastroenterol 2000; 6(5): 688-692
- URL: https://www.wjgnet.com/1007-9327/full/v6/i5/688.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i5.688
Apoptosis is one of the cell death forms, which is quite different from cell necrosis in morphology, biochemistry and biology. Clinical studies showed that ET (Endotoxin, ET) and tumor necrosis factor-α (TNF-α) elevated obviously in the sera of patients with severe hepatitis. Recent researches showed that hepatocyte apoptosis was closely related with pathogenesis of hepatitis especially that of ASH[1-11]. The present study deals with the effect of both apoptosis and necrosis induced by TNF-α on the pathogenesis in ASH, and their relationship.
Recombinant murine TNF-α and immunoglobulin (Ig) G1 fraction of anti-murine TNF-α were purchased from Pepro Tech EC LTD. Terminal-deoxynucleotidyl transferase (TdT) and Bio-11-dUTP Na salt were purchased from Dako LTD. Salmonella abortus equi endotoxin (ET) was purchased from Sigma Chemical Co. GalN was purchased from Chong Qing Medical University. Endogen mouse TNF-α ELISA Kit was provided by Endogen Inc. Unless otherwise specified, all other reagents were analytical reagents.
One hundred and thirty specific pathogen-free male BALB/c mice (from Shanghai Second Medical university animal breeding house) were divided into 7 groups: ① Control; ② GalN; ③ ET; ④ TNF-α; ⑤ GalN/ET; ⑥ GalN/TNF-α; and ⑦ anti-TNF-α IgG1/GalN/ET.
Each group contained 20 mice except Group 7 which contained 10 mice. Both GalN (800 mg/kg) and ET (2.4 μg/kg) were injected intraperitoneally; Both TNF-α (1.0 μg/kg) and anti-TNF-α IgG1 (100 μg/mouse) were injected in tail vein. The control group received the same volume of normal saline.
Five mice were killed at 1.5 h, 3.5 h, 6 h, and 9 h respectively after injection. TNF-α, ALT, and AST were measured in the blood samples taken from the mouse heart. Tissue samples taken from liver were also prepared for morphological and molecular biological examinations.
The sections of liver tissue were stained with hematoxylin and eosin (HE), and ISEL[12] for detection of apoptotic liver cells were performed, DNA was extracted from fresh liver tissue for further analysis of DNA ladder.
Hepatic lobular architecture was clear and intact without any abnomalities in the liver section of control group. Only mild swelling of hepatic cell was presented in GalN, ET and TNF-α groups. With the time prolonging from 1.5 h, 3.5 h, 6 h, to 9 h, the mild swelling become moderate degree. Neither apoptosis nor necrosis was present in HE and ISEL staining. No DNA ladder was found in any group mentioned above.
No obvious liver cell alterations were present at 1.5 h on the section of HE staining and ISEL staining failed to detect positive signals of apoptosis.
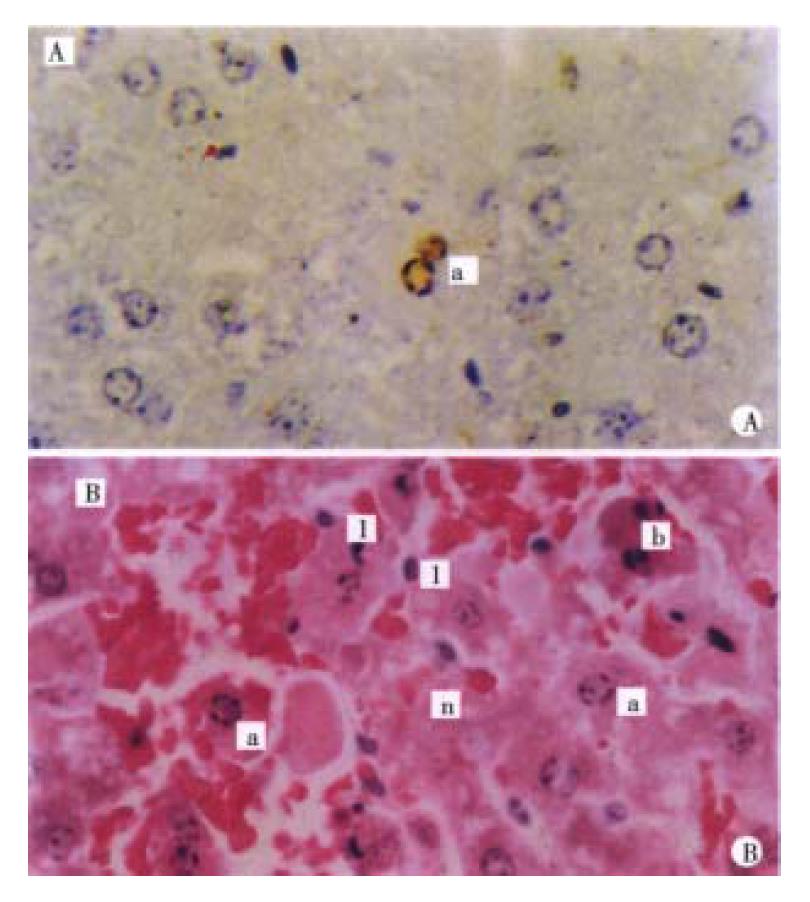
Mild swelling of liver cells on HE section and a few apoptotic cells on ISEL section were present at 3.5 h (Figure 1A).
Obvious swelling of liver cells and lots of apoptotic cells were found at 6 h and mild dotted necrosis was also observed. Strong apoptotic positive signal s could be detected in the cell nuclei.
Nine hours after GalN/ET injection, enormous apoptotic liver cells and pieces of hepatic necrosis with leukocytes infiltration can be seen (Figure 1B).
These data demonstrate that apoptosis occurred as an early event during the development of hepatic failure. Compared with the ET model, the majority of changes occurred earlier in the TNF model, which agrees with the phenomena that ET act on hepatocytes by inducing TNF-α.
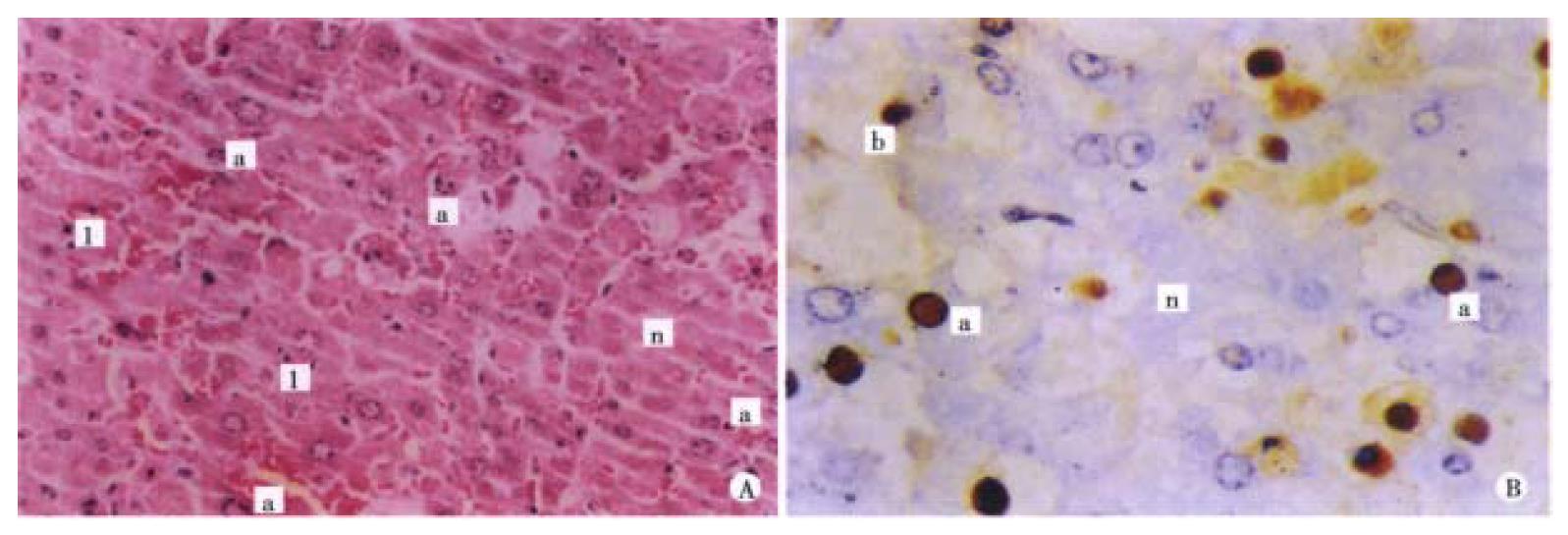
Morphological changes of the liver in this group were basically the same as those in GalN/ET group but severer at 1.5 h, 3.5 h, 6 h and 9 h ( Figure 2A, B), 1.5 h, 3 h, 6 h and 9 h after adiministrating inducer, apoptosis positive rates of GalN/ET group were 0.0%, 0.2%, 1.21% and 3.14% respectively, while for the GalN/TNF-α group, were 0.2%, 0.5%, 2.57% and 3.19% respectively.
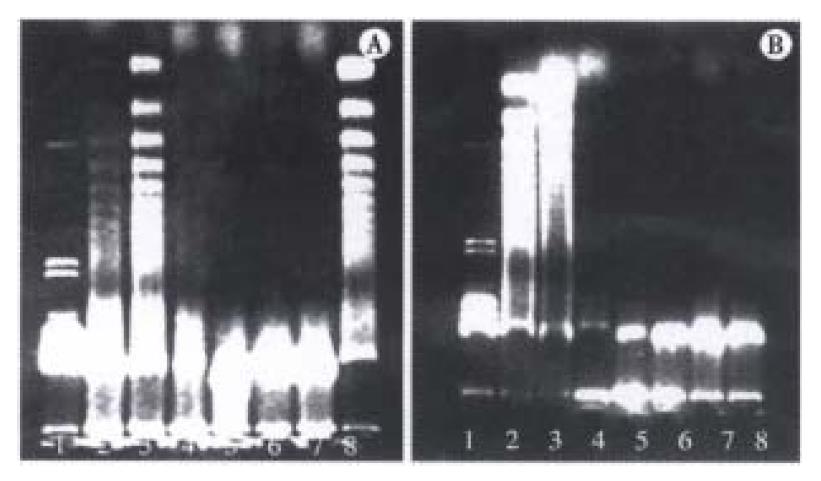
DNA ladder was found at 6 h and 9 h after GalN/ET, GalN/TNF-α adiministration, but was not found at 1.5 h and 3.5 h after GalN/ET, GalN/TNF-α injection, and DNA ladder was not found in other groups (Figure 3A, B).
Electron microscopic study showed the chromatin condensation of apoptotic cells and near the nuclear lining (Figure 4).
In the GalN/ET group pretreated with anti-TNF-α IgG1, only mild swelling of liver cells could be seen on the HE staining. Neither apoptosis nor necrosis could be found on ISEL staining. No DNA ladder was present on electrophoresis of agarose gel (Figure 3A).
We compared the time course of typical morphology of apoptosis (chromatin condensation, apoptotic bodies) with ALT and AST (as a parameter for necrosis) in each group (Table 1). ALT and AST remained normal at 3.5 h after GalN/TNF-α challenge, at this time point, a small amount of apoptotic liver cells could be found. Apoptotic liver cells become obvious at 6 h, ALT and AST were elevated mildly at the same time. This finding indicated that apoptosis was already developed while the liver cell membrane still remained intact 6 h after challenge, suggesting that apoptosis occurred earlier than necrosis.
| 1.5 h | 3.5 h | 6 h | 9 h | |||||||||
| TNF | ALT | AST | TNF | ALT | AST | TNF | ALT | AST | TNF | ALT | AST | |
| 1 | 13.9 ± 15.0 | 39.6 ± 10.3 | 124.0 ± 17.0 | 18.8 ± 15.2 | 40.1 ± 6.0 | 129.6 ± 33.5 | 15.9 ± 15.9 | 41.5 ± 5.4 | 100.1 ± 24.3 | 21.9 ± 16.4 | 41.4 ± 6.6 | 115.6 ± 16.8 |
| 2 | 16.0 ± 16.0 | 40.3 ± 6.0 | 95.7 ± 37.5 | 10.1 ± 16.2 | 40.8 ± 12.8 | 118.4 ± 40.1 | 28.0 ± 21.1 | 46.8 ± 2.41 | 35.4 ± 62.4 | 25.4 ± 29.7 | 47.3 ± 9.0 | 100.2 ± 35.5 |
| 3 | 32.1 ± 14.6 | 53.7 ± 11.2 | 53.1 ± 21.1 | 12.3 ± 17.4 | 43.6 ± 6.5 | 52.4 ± 23.0 | 50.8 ± 43.3 | 39.2 ± 10.4 | 63.0 ± 20.1 | 44.4 ± 31.6 | 47.5 ± 15.4 | 58.2 ± 30.0 |
| 4 | 141.7 ± 96.5a | 36.6 ± 9.8 | 110.9 ± 49.1 | 87.8 ± 51.3 | 47.2 ± 23.5 | 75.2 ± 45.7 | 51.1 ± 25.4 | 52.3 ± 20.4 | 119.4 ± 84.7 | 50.1 ± 19.3 | 56.5 ± 25.6 | 110.1 ± 85.8 |
| 5 | 213.5 ± 81.7a | 47.2 ± 112 | 223.0 ± 85.0 | 184.3 ± 94.0 | 150.1 ± 17.8 | 230.1 ± 82.9 | 147.8 ± 103.1a | 268.3 ± 153.8a | 511.5 ± 412.0a | 119.5 ± 98.4 | 684.3 ± 380.8a | 644.4 ± 164.6abcd |
| 6 | 308.6 ± 89.9a | 89.1 ± 37.1 | 285.3 ± 138.4 | 290.5 ± 109.7a | 127.3 ± 70.1a | 344.5 ± 140.1a | 208.9 ± 107.8a | 540.2 ± 176.2a | 602.1 ± 208.6a | 158.6 ± 77.3a | 978.2 ± 319.8a | 1105.7 ± 714.5abcd |
| 7 | 32.7 ± 18.0 | 46.0 ± 13.4 | 108.6 ± 36.0 | 36.3 ± 27.0 | 48.4 ± 14.7 | 129.8 ± 50.3 | ||||||
The prominent increase of ALT and AST occurred at 9 h, when a great number of necrotic liver cells were observed. Meanwhile, profuse apoptotic liver cells were also present even after the death of mice associated with ASH and eletrophoresis of agarose gel still showed DNA ladder at the final stage.
ASH may be caused by viral infection and drug intoxication. It was believed that the large amount of liver cell death was necrosis due to associated immune damage mediated by dysfunction of host immune system and TNF-α may cause liver necrosis directly[13,14]. Recent studies have shown that besides necrosis, hepatic apoptosis induced by TNF-α plays an important role in the course of ASH[1,15-27]. Our study showed that only mild injury could be found by injecting ET or TNF-α alone. While the combination of GalN with either ET or TNF-α can cause ASH in mice.
Liver cells may synthesize the protecting protein after exposure to injury factors. The process needs the participation of intact cyto-metabolism and protein- synthesis mechanism. GalN may specifically deplete uridine nucleotides in liver cell and influence its metabolic course, leading to a hepatic transcriptional block and the suppression of protecting protein synthesis and then sensitizes the liver cell to TNF-α[28-34].
TNF-α may induce apoptosis of liver cell which is transfected by hepatitis B virus or other virus[35-41], suggesting the cells infected by virus involved in TNF-α sensitivity.
The results of our study showed that TNF-α was mainly produced in the early stage of endotoxemia, and decreased obviously from 6h to 9 h after challenge. TNF-α combined with TNF-α receptor on the membrane of liver cells through a series signal transmssion activating caspase-3 and then inducing apoptosis, and TGF-β1 can also produce similar effect which can induce apoptosis[42-45], delayed treatment with the caspase 3-like protease inhibitor Z-VAD attenuated apoptosis by 81% to 88% and prevented liver cell necrosis[46]. At the same time TNF-α can activate nuclear transc ription factor-κα (NF-κα) of hepatocytes[47], Kupffer cells and endotheliocyte, which increases expression of ICAM-1, VCAM-1 and selectin, these inflamatory factors futher induce the in flamatory injury of hepatocytes, and TNF-α also induce Shwartzman-like reaction in the liver[48]. Recent study demonstrated that mitochondria may be the centre of cell apoptosis, if mitochondrial structural alterations occur without functional failure, the cell dies by apoptosis. In contrast, if the injury is severe enough to lead to mitochondrial functional failure, the cell dies by necrosis[49,50].
In summary, our results showed that TNF-α plays an important role in the course of hepatic apoptosis and necrosis. The blockage of liver apoptotic signal transmission and caspase activation induced by TNF-α with Z-VAD, anti-ET antibody and anti-TNF monoclone antibody can improve prognosis of fulminant hepatic failure[2,42-46,51] and may prevent liver cell from apoptosis and necrosis and hence has an important significance in the prevention and treatment of ASH.
Edited by You DY Verified by Ma JY
| 1. | Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220-1234. [PubMed] |
| 2. | Tsukidate K, Yamamoto K, Snyder JW, Farber JL. Microtubule antagonists activate programmed cell death (apoptosis) in cultured rat hepatocytes. Am J Pathol. 1993;143:918-925. [PubMed] |
| 3. | Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1429] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 4. | Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541-1554. [PubMed] |
| 5. | Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-gamma(-/-) mice, but not in TNF-alpha(-/-) mice: role for IFN-gamma in activating apoptosis of hepatocytes. J Immunol. 1997;159:1418-1428. [PubMed] |
| 6. | Guo LL, Guo Y, Chao CA. Expressions of CD 44/.CD 54 and Fas/.FasL in chronic viral hepatitis. Shijie Huaren Xiaohua Zazhi. 1999;8:705-706. |
| 7. | Okamoto T, Yamakawa T, Yamamura K, Hino O. Induction of Fas ligand and Fas antigen mRNA expressions in interferon-gamma transgenic mouse liver. Jpn J Pharmacol. 1998;78:233-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387-G392. [PubMed] |
| 9. | Rosenfeld ME, Prichard L, Shiojiri N, Fausto N. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am J Pathol. 2000;156:997-1007. [PubMed] |
| 10. | Okamoto T, Nakano Y, Yamakawa T, Hara K, Yamamura KI, Hino O. Chronic hepatitis in interferon-gamma transgenic mice is associated with elevated CPP32-like activity and interleukin-1beta-converting enzyme activity suppression. Jpn J Pharmacol. 1999;79:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995;21:190-198. [PubMed] |
| 12. | Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6746] [Cited by in RCA: 7191] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 13. | Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988;2:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Wang JH, Redmond HP, Watson RW, Bouchier-Hayes D. Role of lipopolysaccharide and tumor necrosis factor-alpha in induction of hepatocyte necrosis. Am J Physiol. 1995;269:G297-G304. [PubMed] |
| 15. | González-Amaro R, García-Monzón C, García-Buey L, Moreno-Otero R, Alonso JL, Yagüe E, Pivel JP, López-Cabrera M, Fernández-Ruiz E, Sánchez-Madrid F. Induction of tumor necrosis factor alpha production by human hepatocytes in chronic viral hepatitis. J Exp Med. 1994;179:841-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 211] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Tiegs G, Niehörster M, Wendel A. Leukocyte alterations do not account for hepatitis induced by endotoxin or TNF alpha in galactosamine-sensitized mice. Biochem Pharmacol. 1990;40:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | ang GQ, Yu H, Zhou XQ, Liao D, Xie Q, Wang B. TNF-α induced apopt osis and necrosis of mice hepatocytes. Shijie Huaren Xiaohua Zazhi. 2000;8:303-306. |
| 18. | Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol. 1994;98:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Fan X, Zhang Z. Increased tumour necrosis factor alpha production by neutrophils in patients with hepatitis B. J Clin Pathol. 1994;47:616-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Zhang GQ, Zhou XQ, Yu H, Xie Q, Wang B, Zhao GM, Guo Q, Xiang YQ, Liao D. The roles of TNF-α induced hepatocyte apoptosis in the development of fulminant liver failure. Zhonghua Xiaohua Zazhi. 2000;20:163-166. |
| 21. | Nakao A, Taki S, Yasui M, Kimura Y, Nonami T, Harada A, Takagi H. The fate of intravenously injected endotoxin in normal rats and in rats with liver failure. Hepatology. 1994;19:1251-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | van Leeuwen PA, Hong RW, Rounds JD, Rodrick ML, Wilmore D. Hepatic failure and coma after liver resection is reversed by manipulation of gut contents: the role of endotoxin. Surgery. 1991;110:169-174; discussion 174-175. [PubMed] |
| 23. | Küsters S, Gantner F, Künstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 353] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Gantner F, Leist M, Jilg S, Germann PG, Freudenberg MA, Tiegs G. Tumor necrosis factor-induced hepatic DNA fragmentation as an early marker of T cell-dependent liver injury in mice. Gastroenterology. 1995;109:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457-467. [PubMed] |
| 26. | Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis,and nitrite release. J Immunol. 1995;154:1307-1316. |
| 27. | Chosay JG, Essani NA, Dunn CJ, Jaeschke H. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin induced injury. Am J Physiol. 1997;272:G1195-1200. |
| 28. | Bahrami S, Redl H, Leichtfried G, Yu Y, Schlag G. Similar cytokine but different coagulation responses to lipopolysaccharide injection in D galactosa mine sensitized versus nonsensitized rats. Infect Immun. 1994;62:99-105. |
| 29. | Seyberth HW, Schmidt-gayk H, Hackental E. Toxicity, clearance and distribution of endotoxin in mice as influnced by actinomycin D, cycloheximide,α -amanitin and lead acetate. Toxico. 1972;10:491-495. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Galanos C, Freudenberg MA, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939-5943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 711] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 31. | Wallach D, Holtmann H, Engelmann H, Nophar Y. Sensitization and desensitization to lethal effects of tumor necrosis factor and IL-1. J Immunol. 1988;140:2994-2999. [PubMed] |
| 32. | Lehmann V, Freudenberg MA, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987;165:657-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 418] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Leist M, Gantner F, Bohlinger I, Germann PG, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol. 1994;153:1778-1788. [PubMed] |
| 34. | Tiegs G, Wolter M, Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1989;38:627-631. [PubMed] |
| 35. | Jiang YG, Li QF, Wang YM, Gu CH. Fas/.FasL expression and hepatocyte apoptosis on liver tissues in tupaia with HDV/HBV infection. Shijie Huaren Xiaohua Zazhi. 2000;8:406-409. |
| 36. | Guilhot S, Miller T, Cornman G, Isom HC. Apoptosis induced by tumor necrosis factor-alpha in rat hepatocyte cell lines expressing hepatitis B virus. Am J Pathol. 1996;148:801-814. [PubMed] |
| 37. | Gilles PN, Guerrette DL, Ulevitch RJ, Schreiber RD, Chisari FV. HBsAg retention sensitizes the hepatocyte to injury by physiological concentrations of interferon-gamma. Hepatology. 1992;16:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Jiang YG, Li QF, Mao Q, Wang YM, Gu CH, Zhang J. ICE expression in hepatocytes in tupaia with HDV/.HBV infection. Shijie Huaren Xiaohua Zazhi. 2000;8:296-298. |
| 39. | Gut JP, Schmitt S, Bingen A, Anton M, Kirn A. Probable role of endogenous endotoxins in hepatocytolysis during murine hepatitis caused by frog virus 3. J Infect Dis. 1984;149:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Mori W, Aoki N, Shiga J. Acute hepatic cell necrosis experimentally produced by viral agents in rabbits. Am J Pathol. 1981;103:31-38. [PubMed] |
| 41. | Bian ZQ, Wang WY, Qin YZ, Xiao RM, Wang GZ, Qin HY. Experimental study of tumor necrosis factor induced acute liver necrosis in duckling infected with DHBV. Zhonghua Chuanranbing Zazhi. 1992;10:88-92. |
| 42. | Okamoto T, Kobayashi T, Tsuzuike N, Hara K. Induction of CPP32-like activity and inhibition of interleukin 1beta converting enzyme activity in the liver of a mouse concanavalin A-induced hepatitis model. Jpn J Pharmacol. 1998;77:257-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Rodriguez I, Matsuura K, Ody C, Nagata S, Vassalli P. Systemic injection of a tripeptide inhibits the intracellular activation of CPP32-like proteases in vivo and fully protects mice against Fas-mediated fulminant liver destruction and death. J Exp Med. 1996;184:2067-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 215] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Inayat-Hussain SH, Couet C, Cohen GM, Cain K. Processing/activation of CPP32-like proteases is involved in transforming growth factor beta1-induced apoptosis in rat hepatocytes. Hepatology. 1997;25:1516-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Cain K, Inayat-Hussain SH, Couet C, Cohen GM. A cleavage-site-directed inhibitor of interleukin-1 beta-converting enzyme-like proteases inhibits apoptosis in primary cultures of rat hepatocytes. Biochem J. 1996;314:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3(CPP32) like proteases is essential for TNF-α -induced hepatic parenchymal cell apoptosis and neutrophil mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480-3486. |
| 47. | Essani NA, McGuire GM, Manning AM, Jaeschke H. Endotoxin induced activation of the nuclear transcription factor κβ and expression of E selectin m essenger RNA in hepatocytes, kupffer cells, and endothelial cells in vivo. J Immunol. 1996;156:2956-2963. |
| 48. | Movat HZ, Burrowes CE, Cybulsky MI, Dinarello CA. Acute inflammation and a shwartzman like reaction induced by interleukin 1 and tumor necrosis factor. Am J Pathol. 1987;129:463-476. |
| 49. | Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6842] [Cited by in RCA: 6813] [Article Influence: 252.3] [Reference Citation Analysis (0)] |
| 50. | Botla R, Spivey JR, Aguilar H, Bronk SF, Gores GJ. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J Pharmacol Exp Ther. 1995;272:930-938. [PubMed] |
| 51. | Manthous CA, Schmidt GA, Kemp R, Wood LD. Fulminant hepatic failure treated with anti-endotoxin antibody. Crit Care Med. 1992;20:1617-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |