Published online Oct 15, 2000. doi: 10.3748/wjg.v6.i5.651
Revised: August 18, 2000
Accepted: August 25, 2000
Published online: October 15, 2000
AIM: To examine whether nizatidine stimulates duodenal HCO3- secretion in rats by inhibiting AChE activity.
METHODS: Under pentobarbital anesthesia, a proximal duodenal loop was perfused with saline, and the HCO3- secretion was measured at pH7.0 using a pH-stat method and by adding 10 mM HCl. Nizatidine, neostigmine, carbachol or famotidine was administered i.v. as a single injection.
RESULTS: Intravenous administration of nizatidine (3-30 mg/kg) dose-dependently increased duodenal HCO3- secretion, and the effect at 10 mg/kg was equivalent to that obtained by carbachol at 0.01 mg/kg. This nizatidine action was observed at the same dose range that inhibited acid secretion and enhanced gastric motility, mimicked by i.v. injection of neostigmine (0.03 mg/kg), and significantly attenuated by bilateral vagotomy and prior s.c. administration of atropine but not by indomethacin, a cyclooxygenase inhibitor, or NG-nitro-L-arginine methyl ester, a NO synthase inhibitor. The HCO3- secretory response to acetylcholine (0.001 mg/kg) was significantly potentiated by the concurrent administration of nizatidine (3 mg/kg, i.v.). The IC50 of nizatidine for AChE of rat erythrocytes was 1.4 × 10-6 M, about 12 times higher than that of neostigmine. Neither famotidine (> 10-3 M, 30 mg/kg, i.v.) nor cisapride (> 10-3 M, 3 mg/kg, i.v.) had any influence on AChE activity or duodenal HCO3- secretion. Duodenal damage induced by acid perfusion (100 mM HCl for 4 h) in the presence of indomethacin was significantly prevented by nizatidine and neostigmine, at the doses that increased the HCO3- secretion.
CONCLUSION: Nizatidine stimulates duodenal HCO3- secretion, in both vagal-dependent and atropine-sensitive manners, and the action is associated with the anti-AChE activity of this agent.
- Citation: Takeuchi K, Kawauchi S, Araki H, Ueki S, Furukawa O. Stimulation by nizatidine, a histamine H2-receptor antagonist, of duodenal HCO3- secretion in rats: relation to anti-cholinesterase activity. World J Gastroenterol 2000; 6(5): 651-658
- URL: https://www.wjgnet.com/1007-9327/full/v6/i5/651.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i5.651
Nizatidine, a histamine H2-receptor antagonist (N-[2-[[[2-[(dimethylamino) methyl]-4-thiazolyl]-methyl] thio] ethyl]-N”-methyl-2-nitro-1, 1-ethenediamine), has been shown to have a potent antisecretory action and clinically ascertained to be effective for pepticulcers as well as gastroesophageal reflux diseases[1-3]. Of interest, an additional effect of H2-antagonists on gastrointestinal motility has been reported, besides the antisecr etory activities[4,5]. Indeed, some H2-antatgonists including nizatidine exhibit a potent anti-acetylcholinesterase (AChE) activity[6-9] and , as a result of this action, facilitate gastrointestinal motor activity in experimental animals and in humans[10,11].
On the other hand, duodenal mucosal HCO3-secretion is a key process that aids in preventing acid-peptic injury[12,13]. The mechanisms that govern mucosal HCO3- secretion include neuro-humoral factors and luminal acid[12-14]. The ability of the mucosa to respond to acid seems especially important in the maintenance of the surface pH gradient and in the protection of mucosa. This process is also mediated by endogenous prostaglandins (PGs) as well as neuronal factors including vagal-cholinergic mechanisms[15-17]. Several investigators reported that cholinomimetic drugs increased duodenal HCO3- secretion, either directly or indirectly mediated by vasoactive intestinal polypeptide (VIP)[17]. We also reported that both carbahol and bethanechol stimulated the HCO3- secretion, mediated by M3 but not M1 receptors[18]. Because inhibition of AChE activity increases the availability of endogenous acetylcholine, it is possible that nizatidine might increase duodenal HCO3- secretion through inhibition of AChE activity. However, the effect of nizatidine on the HCO3- secretion has not been studied.
This study was undertaken to confirm the anti-AChE activity of nizatidine in vitro, and investigate the effect of this agent on HCO3- secretion in the rat duodenum, in comparison with the other H2-antagonist famotidine and the AChE inhibitor neostigmine. In addition, we also examined whether the doses sufficient to stimulate the HCO3-secretion are comparable to the gastropro kinetic and antisecretory doses.
Male Sprague-Dawley rats, weighing 200 g-230 g (Charles River, Shizuoka, Japan), were used in all experiments. The animals kept in individual cages with raised mesh bottoms were deprived of food but allowed free access to tap water for 18 h before the experiments. Studies were carried out with 4-6 rats per group under anesthetized conditions induced by pentobarbital Na (30 mg/kg, i.v.), unless otherwise specified. All experimental procedures described here were approved by the Experimental Animal Research Committee of the Kyoto Pharmaceutical University.
Duodenal HCO3- secretion was determined in the duodenal loop according to a previously published method[16]. In brief, the abdomen was incised, and the stomach and duodenum were exposed. The duodenal loop (1.7 cm) was made between the pylorus and the area just proximal to the outlet of the common bile duct, excluding the influences of bile acid and pancreatic juice. Then, the loop was perfused at a flow rate of 0.8 mL/min with saline that was gassed with 100% O2 and kept in a reservoir, and HCO3- secretion was measured at pH7.0 using a pH-stat method and by adding 10 mM HCl to the reservoir. Allowing 30-40 min for stabilization of basal HCO3- secretion, the duodenal HCO3- secretory responses were examined for 2 h after the following treatment; nizatidine (3-30 mg/kg), carbachol (0.003 mg/kg), neosti-gmine (0.03 mg/kg), famotidine (10 mg/kg) or cisapride (3 mg/kg). These drugs were administered i.v. as a single injection. In some cases, nizatidine (3 mg/kg) and acetylcholine (0.001 mg/ kg) were administered i.v. simultaneously. In some cases, atropine (1 mg/kg, s.c.), indomethacin (5 mg/kg, s.c.) or N-G-nitro-L-arginine methyl ester (L-NAME) the NO synthase inhibitor (5 mg/kg, i.v.) was given 1 h or 10 min, respectively, before administration of nizatidine, carbachol or neostigmine. In a separate experiment, bilateral vagotomy was performed at the cervical portion 2 h before administration of nizatidine, neostigmine or carbachol.
Gastric acid secretion was measured in a chambered stomach, according to a previously published method[19]. Briefly, the abdomen was incised, and both the stomach and duodenum were exposed. Then, the stomach was mounted in an ex-vivo chamber and perfused at a flow rate of 0.8 mL/min with saline that was gassed with 100% O2, heated at 37 °C and kept in a reservoir. The acid secretion was measured at pH7.0 using a pH-stat method (Hiranuma Comtite-8, Tokyo, Japan) by adding 100 mM NaOH to the reservoir. After basal acid secretion had well stabilized, the acid secretion was stimulated by continuous i.v. infusion of histamine (4 mg/kg/h). Nizatidine (10 and 30 mg/kg) and famotidine (10 mg/kg) were administered i.v. as a single injection 1h after the onset of histamine infusion, when the acid secretory response to histamine had reached a plateau.
Gastric motility was determined using a miniature balloon in conscious rats, according to a previously published method[20]. Briefly, under ether anesthesia the balloon and the support catheter were placed in the stomach through an incision of the forestomach. The animals were kept in Bollman cages, and gastric motility was monitored on a Hitachi recorder (Model 056, Mito, Japan) using a pressure transducer (Narco Telecare, Model 151-T, Houston, TX., U.S.A.) and a polygraph device (San-ei, Model 6M-72, Tokyo, Japan) after complete recovery from anesthesia. After basal motility had well stabilized, the animals were administered i.v. with nizatidine (30 mg/kg), neostigmine (0.03 mg/kg), cisapride (3 mg/kg) or famotidine (10 mg/kg), and the motility was measured for 2 h thereafter. In some cases, the effect of atropine (1 mg/kg, s.c.) was examined on the enhanced gastric motility in response to nizatidine (30 mg/kg, i.v.).
The increased HCO3- secretion caused by nizatidine might lead to decrease of the mucosal susceptibility to acid injury. To test this possibility, we examined the effect of nizatidine on the mucosal ulcerogenic response by perfusing the duodenum with 100 mM HCl at the flow rate of 1 mL/h for 4 h in the presence of indomethacin. The experiment was performed in the duodenal loop preparation, similar to that described for HCO3- secretion. Animals were first treated with indomethacin (5 mg/kg, s.c.), and 1 h later the duodenum was perfused with acid for 4 h. Nizatidine (10 mg/kg) was administered i.v. 20 min before the onset of acid perfusion. In comparison, the animals were treated i.v. with neostigmine (0.03 mg/kg) or famotidine (10 mg/kg). The duodenums were removed, inflated by injecting 0.5 mL of 2% formalin, immersed in 2% formalin for 10 min to fix the tissue wall, opened along the mesenteric artery, and examined for lesions under a dissecting microscope with a square grid (× 10). The area (mm2) of each lesion was measured, summed per duodenum, and used as a damage score. The person measuring the lesions did not know the treatment given to the animals.
Erythrocyte membranes and plasma each from rats were prepared to obtain both AChE and pseudocholinesterase (PChE), according to the method described by Hansen and Bert[6]. Before the determination of anti-ChE activity, it was confirmed that PChE was not present in the erythrocyte membranes by using the PChE inhibitor, profenamine. The anti-ChE activity of H2-receptor antagonists and neostigmine was determined by the modified method of Ellman et al[21]. In brief, a reaction mixture was prepared to contain, in a total volume of 1 mL, 0.1 M sodium phosphate buffer (pH8.0), 1 mM acetylcholine, 0.3 mM 5,5-dithio-bis (2-nitrobenzoic acid) and 1 mU ChE. The enzyme activity was determined by tracing the changes in absorbance at 412 nm at 30 °C on a spectrophotometer (Model 320, Hitachi, Ibaragi, Japan) for a period of 70 sec after adding acetylthiochlone. The amount of enzyme required to convert 1 μmol of acetylthiocholine within 1 min under the above conditions was taken as 1 unit. Anti-enzyme activity was measured by adding 10 μL of each test drug solution to the reaction mixture. The concentration of test drug required to inhibit 50% of the enzyme activity (IC50) was calculated from the enzyme inhibition curve.
Drugs used were pentobarbital Na, neostigmine bromide, histamine 2HCl, carbachol, acetylcholine (Nacalai tesque, Kyoto, Japan), nizatidine (Zeria Pharm. Co., Saitama, Japan), famotidine (Gaster R, Yamanouchi Pharm. Co. Tokyo, Japan), atropine, indomethacin, NG-nitro-L-arginine methyl ester (Sigma Chemicals, Saint Louis, Mo., USA) and cisapride (Synthesized by Zeria Pharm. Co.). For in vivo experiments, each drug except indomethacin was dissolved in or diluted with saline. Indomethacin was suspended in saline with a drop of Tween 80 (Wako, Osaka, Japan). Each drug was administered i.v. in a volume of 1 mL/kg or s.c. in a volume of 5 mL/kg, or by i.v. infusion in a volume of 1.2 mL/h. For in vitro experiments, each drug was prepared in purified water or equimolar hydrochloric acid solution. In all experiments, solvents alone were used as controls.
Data are presented as the means ± SE from 4-6 rats per group. Statistical analyses were performed using a two-tailed Dunnett’s multiple comparison test, and values of P < 0.05 were regarded as significant.
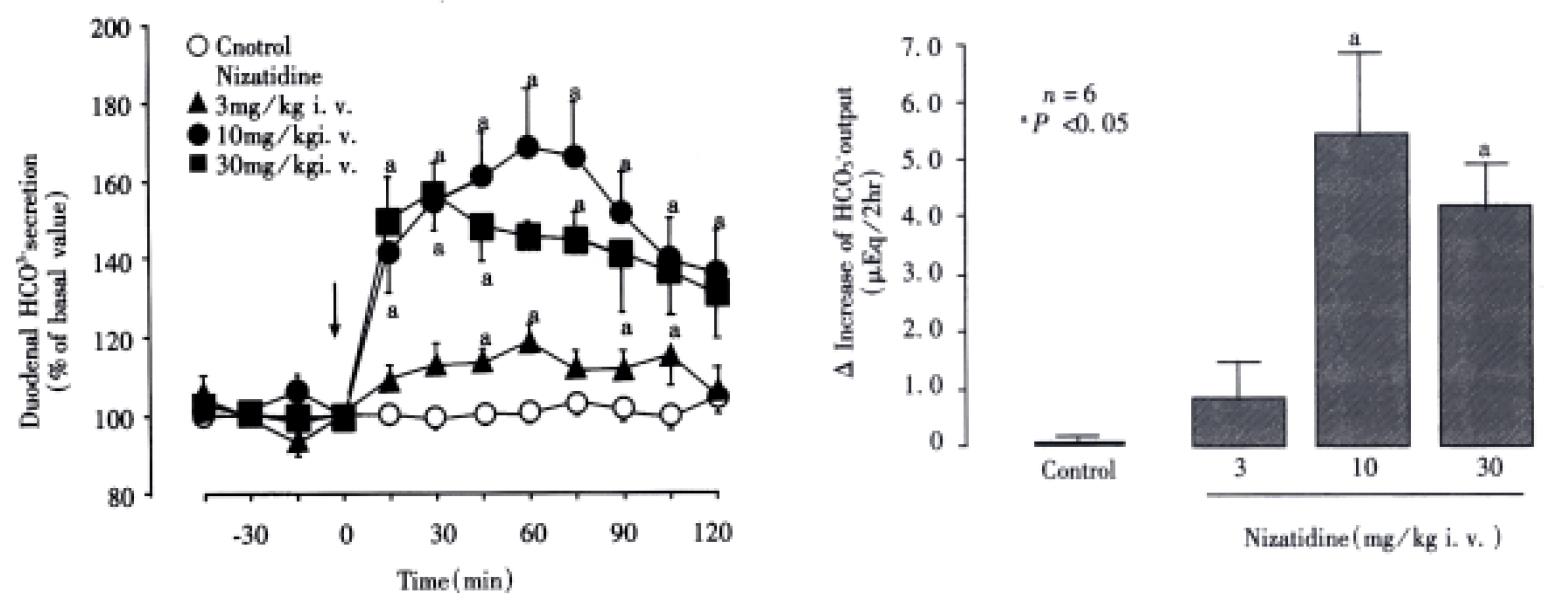
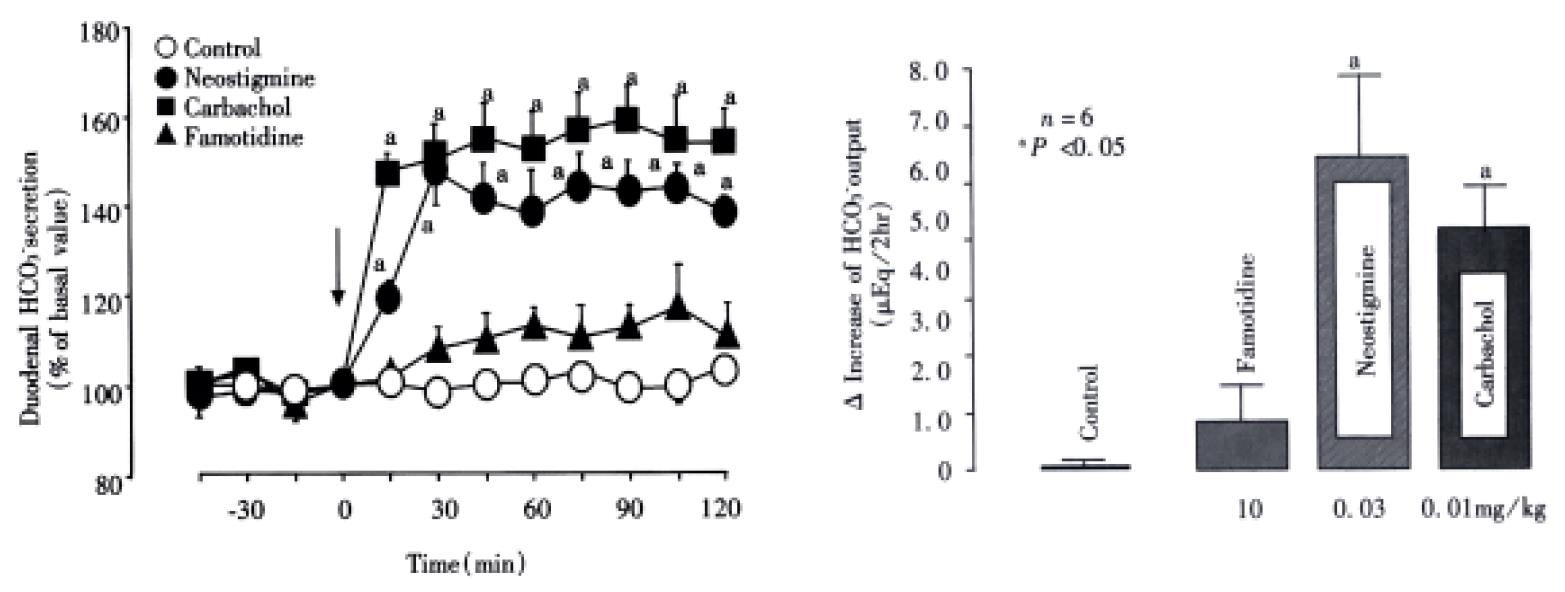
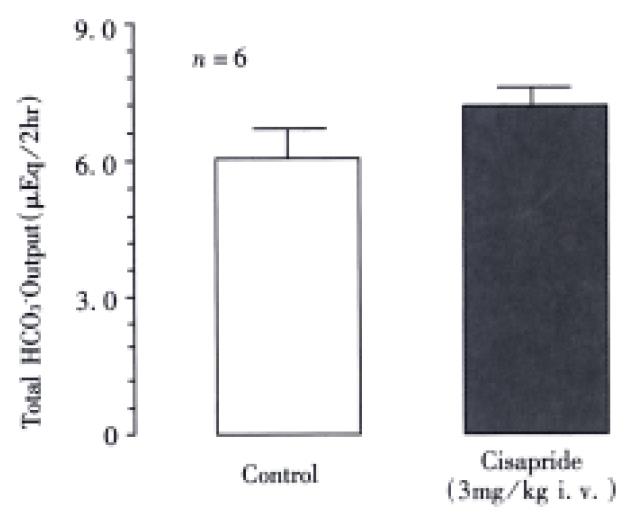
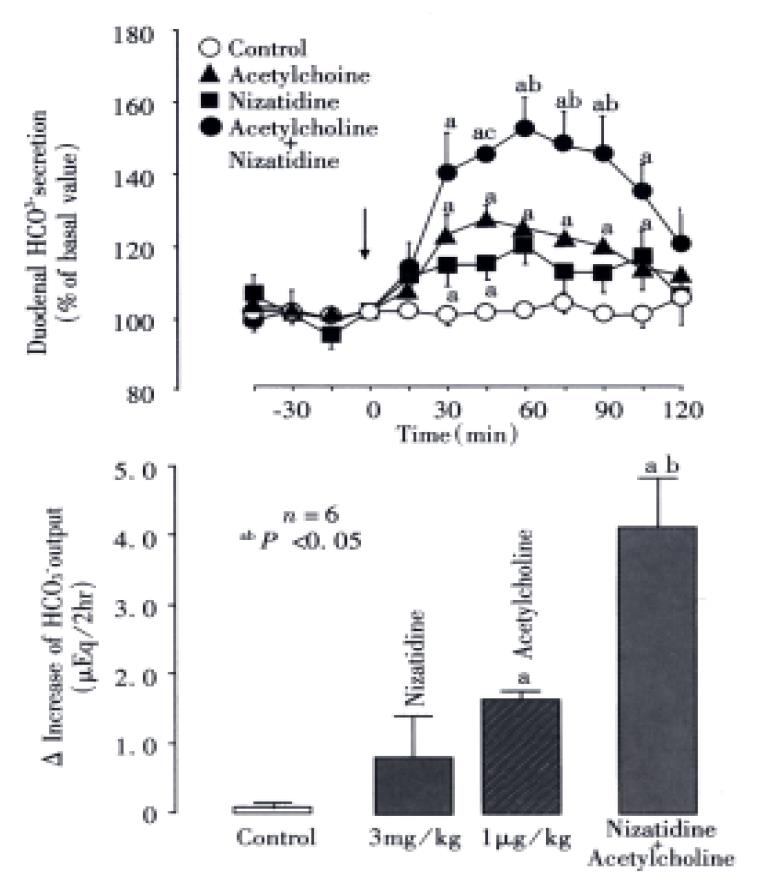
Under the present experimental conditions, the rat duodenum spontaneously secreted HCO3- at a steady rate of 1.0-1.2 μEq/15 min during a 3 h test period. Intravenous administration of nizatidine (3-30 mg/kg) caused an increase of the HCO3-secretion in a dose-dependent manner (Figure 1). At 10 mg·kg-1, the H2 antagonist nizatidine increased the △HCO3- secretion from 1.2 Eq/15 min to a plateau level of 1.8-2.0 Eq/15 min within 30 min, remaining elevated for 2 h, the HCO3- output being 5.6 ± 1.4 Eq/2 h. Likewise, duodenal △HCO3- secretion was significantly increased in response to i.v. administration of carbachol (0.01 mg/kg) and neostigmine (0.03 mg/kg), the HCO3- output being 4.9 ± 1.1 Eq/2 h and 5.2 ± 0.8 Eq/2 h, respectively, both of which were almost equivalent to that induced by nizatidine at 10 mg/kg (Figure 2). By contrast, neither famotidine the H2-antagonist (10 mg/kg, i.v.) nor cisapride the gastroprokinetic drug (3 mg/kg, i.v.) had any influence on basal rates of duodenal HCO3-secretion (Figure 2 and Figure 3).
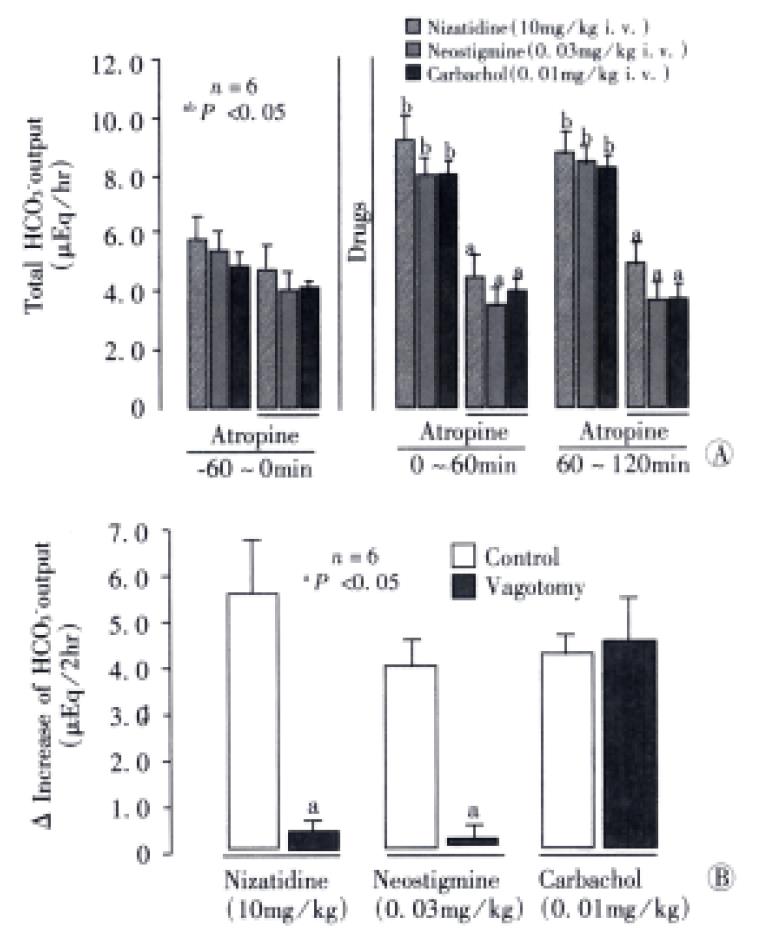
The HCO3- secretory responses induced by both nizatidine (10 mg/kg, i.v), neostigmine (0.03 mg/kg, i.v.) and carbachol (0.01 mg/kg, i.v.). Were all significantly inhibited by prior s.c. administration of atropine (1 mg/kg) (Figure 4). This agent had a minimal effect on the basal HCO3-secretion without any treatment, but almost totally attenuated the increase of HCO3- secretion induced by either nizatidine, neostigmine or carbachol; the △HCO3- output remained unchanged before and at all time points after administration of these drugs. Likewise, bilateral vagotomy significantly reduced the HCO3- secretory response to nizatidine and neostigmine but not carbachol. On the other hand, the pretreatment of tie animals with neither indomethacin (5 mg/kg, s.c.) nor L-NAME (5 mg/kg, i.v.) significantly affected the HCO3- secretory response induced by nizatidine (not shown).
To further investigate the relation of anti-AChE activity of nizatidine with the HCO3- stimulatory action, we examined whether or not the acetylcholine-induced HCO3- response was potentiated by co-administration of nizatidine. As shown in Figure 5, acetylcholine (0.001 mg/kg) caused a slight but significant increase in duodenal HCO3- secretion, while nizatidine at 3 mg·kg-1 tended to increase the secretion; the △HCO3- output was 1.8 ± 0.1 Eq/2 h and 0.7 ± 0.6 Eq/2 h, respectively. However, when nizatidine was administered together with acetylcholine, the HCO3- secretion was markedly increased, reaching a peak of about 150% of basal values, the △HCO3- output being 4.1 ± 0.7 μEq/2 h, which is 2.4 fold greater than that induced by acetylcholine.
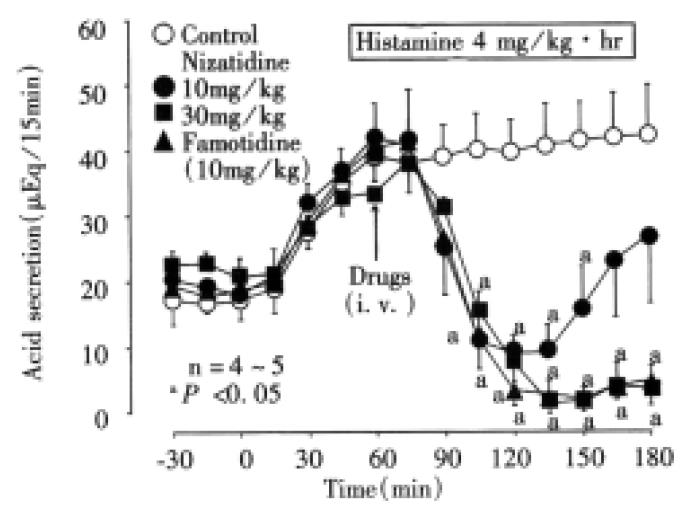
Acid secretion Following intravenous infusion of histamine (4 mg/kg/h), gastric acid secretion was increased from 18.6 ± 3.1 μEq/10 min to 39.5 ± 3.2 μEq/10 min-within 60 min, and remained elevated during a 2 h test period. The acid secretory response to histamine was significantly reduced by i.v. injection of nizatidine (10 and 30 mg/kg) in a dose-dependent manner, the inhibition of total acid output for 2 h being 58.9 and 86.3%, respectively (Figure 6). A potent inhibition of histamine-induced acid secretion was also observed on i.v. administration of famotidine (10 mg/kg), the inhibition of total acid output for 2 h being 90.4%.
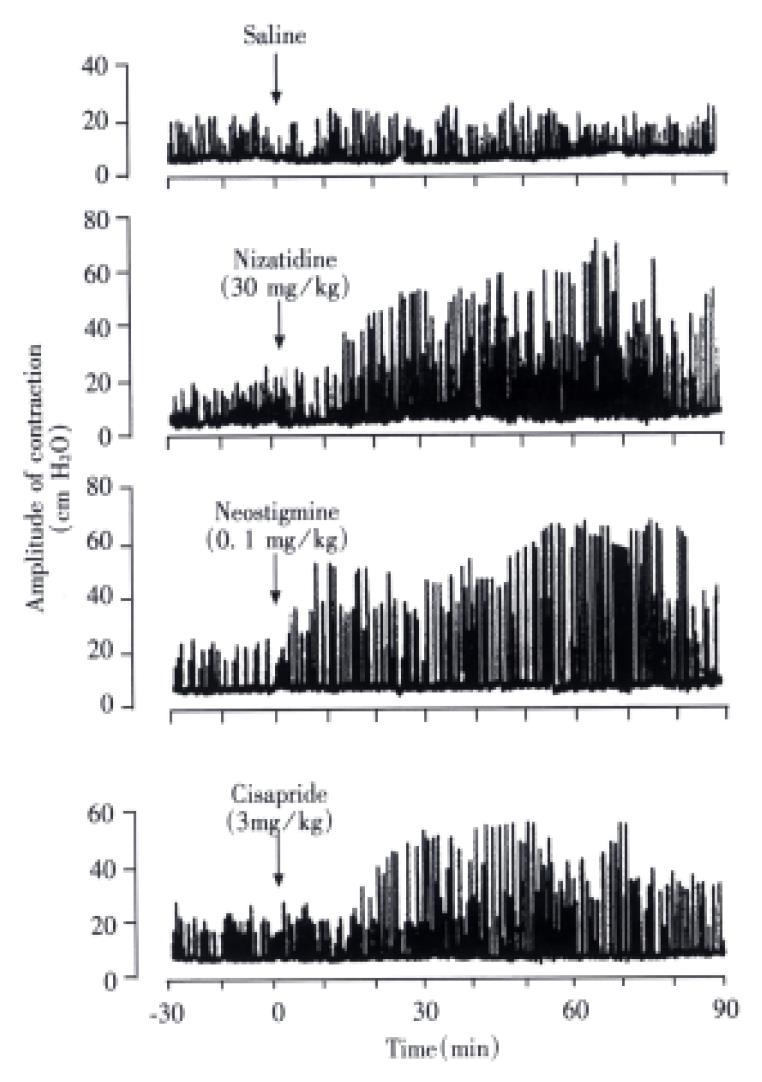
Gastric motility Normal rat stomachs spontaneously contracted at a frequency of 16-20/10 min with an amplitude of 18.6 ± 3.2 cm H2O. Intravenous administration of nizatidine (30 mg/kg) enhanced gastric motility, which reached a plateau level (about 2.5 times greater than basal values) within 40 min and remained elevated thereafter, and this action was completely inhibited by atropine (1 mg/kg, s.c.) (Figure 7). Both neostigmine (0.03 mg/kg) and cisapride (3 mg/kg) increased gastric motility, similar to nizatidine, while famotidine (10 mg/kg) did not have any effect on spontaneous contractile activity of the stomach (not shown).
Perfusion of the proximal duodenum with 100 mM HCl for 4 h in indomethacin-treated rats caused severe damage in the mucosa, the lesion score being 49.1 ± 7.4 mm2 (Table 1). Pretreatment of animals with nizatidine (10 mg/kg, i.v.) or neostigmine (0.03 mg/kg, i.v.) was effective in significantly reducing the severity of duodenal damage in response to acid perfusion, the inhibition being 56.6% or 64.0%, respectively. Famotidine (10 mg/kg, i.v.) had no effect on the development of duodenal damage induced by acid perfusion.
Table 2 summarizes the activities of nizatidine for AChE and PChE, in comparison with neostigmine and famotidine. Nizatidine inhibited the AChE activity prepared from rat erythrocytes, and the IC50 was 1.4 × 10-6 M, about 12 times greater than that (1.1 × 10-7 M) of neostigmine. Likewise, both nizatidine and neostigmine inhibited the PChE activity prepared from rat plasma, the IC50 being 5.7 × 10-4 M and 3.3 × 10-6 M, respectively. However, neither famotidine nor cisapride had any effect on AChE or PChE activities, the IC50 for these drugs being over 1 × 10-3 M.
| Drugs | IC50 (M) | |
| Rat erythrocytes | Plasma | |
| Nizatidine | 1.4 × 10-6 | 5.7 × 10-4 |
| Neostigmine | 1.1 × 10-7 | 3.3 × 10-6 |
| Famotidine | 1.0 × 10-3 < | 1.0 × 10-3 < |
| Cisapride | 1.0 × 10-3 < | 1.0 × 10-3 < |
The present study showed for the first time that nizatidine, an histamine H2-receptor antagonist, stimulates duodenal HCO3- secretion in rats, in both vagal-dependent and atropine-sensitive manners, and this action is associated with the anti-AChE activity of this agent. Furthermore, the HCO3- stimulatory property if nizatidine was observed at the dose ranges for both gastric antisecretory and prokinetic actions. Since neither famotidine nor cisapride had any effect on duodenal HCO3- secretion, it is unlikely that this action of nizatidine is simply resulted from the inhibition of gastric acid secretion due to H2 receptor blockade or the increased luminal pressure due to enhanced duodenalmotility.
It has been shown that several H2-receptor antagonists are endowed with anti-AChE activity[6-10]. This action results in facilitating gastrointestinal motor activity in experimental animals and in humans[5,11]. Ueki et al[11] reported that nizatidine stimulates gastrointestinal motility and gastric emptying at antisecretory doses, mainly through its anti-AChE activity. In the present study, we confirmed that nizatidine potently inhibited both AChE and PChE activities. On the basis of the Ki values, it was also noted that the anti-AChE activity of nizatidine was much potent as compared with the other H2-antagonist famotidine, although it was weaker than that of neostigmine, the authentic AChE inhibitor.
It is widely accepted that acetylcholine is a transmitter released by enteric excitatory neurons to influence gastrointestinal motility. Likewise, several studies showed that duodenal HCO3- secretion was increased by the vagal-cholinergic excitation[17,18,22,23]. Lenz et al[22] reported that the HCO3- secretion was increased in response to both central and peripheral cholinergic agents. It is also known that the vagal-cholinergic pathway plays a role in the HCO3- secretory response to acid and sham feeding[23]. Since the electrical stimulation of the vagus nerves increased duodenal HCO3- secretory mediated by the atropine-sensitive cholinergic pathway[24], it is expected that this process is also stimulated by peripheral cholinomimetic agents. The present study clearly showed that nizatidine increased the HCO3- secretory by stimulating cholinergic excitation through anti-AChE activity, and this effect was mimicked by neostigmine. These results support the contention that the HCO3- stimulatory effect of nizatidine in the rat duodenum is mediated by endogenous acetylcholine released from cholinergic neurons. Indeed, this action of nizatidine on the HCO3- secretion was totally abolished by bilateral vagotomy or prior administration of atropine. Certainly, the antagonism of H2-receptor does not account for the HCO3- stimulatory action of nizatidine, because famotidine did not have any effect on this secretion.
In the present study, the HCO3- secretion induced by nizatidine was not affected by either indomethacin or L-NAME, excluding the possibility for involvement of PG or NO in this response. We previously reported that the HCO3- response induced by cholinergic agents was not affected by indomethacin[18]. Furukawa et al[25] reported that NO stimulates the HCO3- secretion, mediated by endogenous PGs in isolated bullfrog duodenums. Thus, it is reasonable that the HCO3- response induced by nizatidine is not affected by the NO synthase inhibitor L-NAME. Segawa et al[26] reported that nizatidine did not affect in vitro PGE2 biosynthesis and even doses that markedly inhibit gastric acid secretion had no effect on the mucosal PGE2 contents in rat stomachs. Hallgren et al[27] found that the NO synthase inhibitors such as L-NAME caused an increase of both luminal alkalinization and luminal pressure in the rat duodenum and suggested that the HCO3- stimulatory action of L-NAME is due to the neural reflex resulting from the rise in luminal pressure through mechano-receptors. The same mechanism might be applied to the HCO3- stimulatory action of nizatidine, because this agent enhanced gastrointestinal motility, leading to increase of the luminal pressure. However, cisapride at the dose that clearly enhanced gastric motility did not affect the HCO3- secretion. Thus, it is unlikely that nizatidine stimulates duodenal HCO3- secretion due to the increase of luminal pressure, resulting from the smooth muscle contraction.
The secretion of HCO3- in the duodenum is the main defense mechanism against acid. Mucus adherent to the luminal surface of the mucosa provides a zone of low turbulence, allowing the development of a gradient for HCO3- from the luminal side[12,13,28]. Small amounts of HCO3- protect the mucosa against large amounts of acid by neutralizing H+ ions that diffuse back into the mucus layer[13]. A number of studies demonstrated a close relationship between the mucosal ulcerogenic response and the duodenal HCO3- disorder[12,13,29]. Indeed, it is reported that patients with inactive duodenal ulcers have decreased production of HCO3- in the proximal duodenum during exposure to acid[30], though the exact mechanism involved remains unknown. In the present study, perfusion of the proximal duodenum with 100 mM HCl for 4 h produced extensive hemorrhagic damage in rats in the presence of indomethacin. However, nizatidine reduced the severity of duodenal damage at the dose which stimulated HCO3- secretion. These results confirmed that nizatidine afforded protection of the duodenal mucosa by increasing HCO3- secretion, in a PG-independent pathway.
It is known that the mucosal acidification increases the HCO3- secretion via both humoral and neural factors as well as endogenous PGs[12,23,31]. Vasoactive intestinal polypeptide (VIP) is the most likely humoral factor mediating the HCO3- secretory response to acid, because it is a potent stimulant of duodenal HCO3- secretion and is released from nerve endings during the exposure of duodenal mucosa to acid[17]. Odes et al[32] reported that acetylcholine increases a release of VIP from enteric nerves via both muscarinic M1 and M3 receptors, which in turn stimulates HCO3- secretion in the duodenum. Since nizatidine significantly potentiated the HCO3- response to acetylcholine, it is possible that the acid-induced HCO3- secretion is also enhanced by the anti-AChE activity in the presence of nizatidine. Ueki et al[11] demonstrated that the ED50 values of nizatidine for inhibition of acid output in rats overlapped the effective doses for stimulation of gastrointestinal motility and gastric emptying. We also showed that nizatidine increased duodenal HCO3- secretion at the doses that were effective in inhibiting histamine-induced acid secretion or in stimulating gastric contractile activity. These results suggest that the HCO3- stimulatory effect of nizatidine can be expected in duodenal ulcer patients who are treated with this drug at the antisecretory dose.
In summary, the present results clearly showed that the histamine H2-receptor antagonist nizatidine stimulates duodenal HCO3- secretion, and this action is associated with its anti-AChE activity and mediated by vagal-cholinergic mechanisms. It is assumed that the HCO3- stimulatory action of nizatidine is useful for treatment of duodenal ulcer, in addition to its anti-acid secretory and gastric prokinetic effects.
Edited by Ma JY
| 1. | Lazzaroni M, Bianchi Porro G. The effect of an oral morning dose of nizatidine and ranitidine on gastric acid secretion in duodenal ulcer patients. Hepatogastroenterology. 1989;36:490-493. [PubMed] |
| 2. | Fullarton GM, Macdonald AM, McColl KE. Rebound hypersecretion after H2-antagonist withdrawal--a comparative study with nizatidine, ranitidine and famotidine. Aliment Pharmacol Ther. 1991;5:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Parente F, Bianchi Porro G. Acid inhibitory characteristics of nizatidine in man: an overview. Scand J Gastroenterol Suppl. 1994;206:3-7. [PubMed] |
| 4. | Bertaccini G, Scarpignato C. Histamine H2-antagonists modify gastric emptying in the rat. Br J Pharmacol. 1982;77:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | McCallum RW, Prakash C, Campoli-Richards DM, Goa KL. Cisapride. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use as a prokinetic agent in gastrointestinal motility disorders. Drugs. 1988;36:652-681. [PubMed] |
| 6. | Hansen WE, Bertl S. The inhibition of acetylcholinesterase and pseudocholinesterase by cimetidine. Arzneimittelforschung. 1983;33:161-163. [PubMed] |
| 7. | Kounenis G, Voutsas D, Koutsoviti-Papadopoulou M, Elezoglou V. Inhibition of acetylcholinesterase by the H2-receptor antagonist nizatidine. J Pharmacobiodyn. 1988;11:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Bemis K, Bendele A, Clemens J, Deldar A, Gidda J, Hamelink J, Holland D, Lamishaw B, McGrath J, Shannon H. General pharmacology of nizatidine in animals. Arzneimittelforschung. 1989;39:240-250. [PubMed] |
| 9. | Laine-Cessac P, Turcant A, Premel-Cabic A, Boyer J, Allain P. Inhibition of cholinesterases by histamine 2 receptor antagonist drugs. Res Commun Chem Pathol Pharmacol. 1993;79:185-193. [PubMed] |
| 10. | Kosh JW, Sowell JW, Chapman JM. A comparison of the cholinergic activity of selected H2-antagonists and sulfoxide metabolites. Pharm Res. 1989;6:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Ueki S, Seiki M, Yoneta T, Aita H, Chaki K, Hori Y, Morita H, Tagashira E, Itoh Z. Gastroprokinetic activity of nizatidine, a new H2-receptor antagonist, and its possible mechanism of action in dogs and rats. J Pharmacol Exp Ther. 1993;264:152-157. [PubMed] |
| 12. | Flemstrm G. Gastric and duodenal mucosal secretion of bicarbonate. In: Physiology of the Gastrointestinal Tract, Johnson LR (ed). 3rd edition, Raven Press, New York 1994; 1285-1309. |
| 13. | Takeuchi K, Okabe S. Gastroduodenal bicarbonate secretion: Pharmacologi-cal regulation and contribution to mucosal protection. In "Regulatory Mecha-nisms in Gastrointestinal Function", edited by Gaginella TS. CRC Press 1995:1-26. . |
| 14. | Flemstrom G, Garner A. Gastroduodenal HCO3(-) transport: characteristics and proposed role in acidity regulation and mucosal protection. Am J Physiol. 1982;242:G183-G193. [PubMed] |
| 15. | Heylings JR, Garner A, Flemström G. Regulation of gastroduodenal HCO-3 transport by luminal acid in the frog in vitro. Am J Physiol. 1984;246:G235-G242. [PubMed] |
| 16. | Takeuchi K, Tanaka H, Furukawa O, Okabe S. Gastroduodenal HCO3-secretion in anesthetized rats: effects of 16,16-dimethyl PGE2, topical acid and acetazolamide. Jpn J Pharmacol. 1986;41:87-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Hogan DL, Yao B, Steinbach JH, Isenberg JI. The enteric nervous system modulates mammalian duodenal mucosal bicarbonate secretion. Gastroenterology. 1993;105:410-417. [PubMed] |
| 18. | Takeuchi K, Niida H, Okabe S. Characterization of alkaline response induced by cholinergic agents in the rat duodenum: involvement of M2 receptors and the calcium-dependent process. J Pharmacol Exp Ther. 1990;254:465-470. [PubMed] |
| 19. | Kato S, Korolkiewicz R, Rekowski P, Szyk A, Sugawa Y, Takeuchi K. Inhibition of gastric acid secretion by galanin in rats. Relation to endogenous histamine release. Regul Pept. 1998;74:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Takeuchi K, Kato S, Hirata T, Nishiwaki H. Gastric motility and mucosal ulcerogenic responses induced by prokinetic drugs in rats under prostaglandin-deficient conditions. Dig Dis Sci. 1997;42:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Ellman GL, Courtney KD, Andres V, Feather-stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18200] [Cited by in RCA: 18590] [Article Influence: 688.5] [Reference Citation Analysis (0)] |
| 22. | Lenz HJ, Vale WW, Rivier JE. TRH-induced vagal stimulation of duodenal HCO-3 mediated by VIP and muscarinic pathways. Am J Physiol. 1989;257:G677-G682. [PubMed] |
| 23. | Feldman M. Gastric H+ and HCO3- secretion in response to sham feeding in humans. Am J Physiol. 1985;248:G188-G191. [PubMed] |
| 24. | Takehara K, Tashima K, Takeuchi K. Alterations in duodenal bicarbonate secretion and mucosal susceptibility to acid in diabetic rats. Gastroenterology. 1997;112:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Furukawa O, Kitamura M, Sugamoto S, Takeuchi K. Stimulatory effect of nitric oxide on bicarbonate secretion in bullfrog duodenums in vitro. Digestion. 1999;60:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Segawa Y, Takei M, Omata T, Tsuzuike N, Yamaji Y, Kurimoto T, Ozeki M, Tagashira E. Effects of the new anti-ulcer drug nizatidine on prostaglandins in the rat gastric mucosa. Arzneimittelforschung. 1991;41:950-953. [PubMed] |
| 27. | Hällgren A, Flemström G, Sababi M, Nylander O. Effects of nitric oxide inhibition on duodenal function in rat: involvement of neural mechanisms. Am J Physiol. 1995;269:G246-G254. [PubMed] |
| 28. | Takeuchi K, Magee D, Critchlow J, Matthews J, Silen W. Studies of the pH gradient and thickness of frog gastric mucus gel. Gastroenterology. 1983;84:331-340. [PubMed] |
| 29. | Takeuchi K, Furukawa O, Tanaka H, Okabe S. A new model of duodenal ulcers induced in rats by indomethacin plus histamine. Gastroenterology. 1986;90:636-645. [PubMed] |
| 30. | Isenberg JI, Selling JA, Hogan DL, Koss MA. Impaired proximal duodenal mucosal bicarbonate secretion in patients with duodenal ulcer. N Engl J Med. 1987;316:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 160] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Takeuchi K, Matsumoto J, Ueshima K, Okabe S. Role of capsaicin-sensitive afferent neurons in alkaline secretory response to luminal acid in the rat duodenum. Gastroenterology. 1991;101:954-961. [PubMed] |
| 32. | Odes HS, Muallem R, Reimer R, Beil W, Schwenk M, Sewing KF. Cholinergic regulation of guinea pig duodenal bicarbonate secretion. Am J Physiol. 1993;265:G270-G276. [PubMed] |