Published online Oct 15, 2000. doi: 10.3748/wjg.v6.i5.647
Revised: June 22, 2000
Accepted: June 29, 2000
Published online: October 15, 2000
- Citation: Li Y, Su JJ, Qin LL, Yang C, Luo D, Ban KC, Kensler T, Roebuck B. Chemopreventive effect of oltipraz on AFB1-induced hepatocarcinogenesis in tree shrew model. World J Gastroenterol 2000; 6(5): 647-650
- URL: https://www.wjgnet.com/1007-9327/full/v6/i5/647.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i5.647
Hepatocellular carcinoma (HCC) is one of the major cancers in the world with a mortality of more than 250000 cases yearly. More than 137000 cases of HCC were diagnosed each year in China, which acount approximately for more than 40 percent of the total number in the world. HCC has become the second major cause of death for cancer in China since 1990, and its annual mortality is expected to be 21.2 cases per 100000 population in the year 2000. Even though progresses have been achieved for HCC diagnosis and treat ment, its 5-year mortality is still higher than 95 percent[1-3].
The prevalence of HCC is quite different among different areas around the world[4,5]. It is considerably high in South-East Asia and sub-Saharan Africa, particularly in some southern and eastern regions inside China such as Fusui County in Guangxi Zhuang Autonomous Region and Qidong City in Jiangsu Province[6-9]. The standardized incidence of HCC in these high-risk regions may exceed 100 cases per 100000 of population[10]. The obvious difference in geographic distribution of HCC indicates that there must be environmental factors for its pathogenesis.
Aflatoxin B1 (AFB1), which is produced by some strains of Aspergillus flavus, is a potent hepatotoxigen and hepatocarcinogen[11,12], and is considered as a major cause of HCC in some regions[13-18]. It has also been postulated that chronic infection with hepatitis B virus (HBV) in combination with exposure to AFB1 in the diet may contribute to the extraordinary high risk of human HCC in some areas. Actually, two case-control studies in Shanghai have demonstrated a strong interaction between HBV and AFB1 for risk of HCC[14,15]. A similar chemical-viral interaction has been observed in Taiwan[18-20]. The synergism between virus and mycotoxic carcinogen for the development of human HCC suggests that reduction in both risk factors may bring important public health consequences.
The concept of chemoprevention of cancer is over 40 years old and a number of works have been done in this field[21,22]. Looking for effective and safe reagents against AFB1 and/or other HCC related risk factors is one of the most important chemoprotective strategies for HCC[23-26]. Green tea was identified years ago as one of the effective chemopreventive reagents against HCC through a series of animal experiments as well as a clinical trial[27,28]. Recently oltipraz, another preventive agent which was previously described as a potent inhibitor of AFB1 induced hepatocarcinogenesis in rat[29-33], has been shown to inhibit the bioactivation of aflatoxin and enhance its detoxification in a clinical trial[34-37] as well as in human hepatocytes in primary culture[38]. Meanwhile, a universal vaccination program against HBV that started a decade ago now results in lower rates of HCC in children[39]. An experimental model to test the synergistic effect of these two agents and their prevention, therefore, is needed.
Tree shrew (Tupaia spp.) is a kind of small, squirrel-like mammals. Formerly it was considered to belong to the Primate order; currently it is classified into a separate order Scandentia and is supposed to be more closely related to human being than rodents[40,41]. They have been used in biomedical researches since as early as the 1960s. Many researches have been done on its visual and nervous systems. In 1976, however, Reddy et al[42] successfully induced liver cancer in tree shrew by AFB1. Yan et al[43,44] reported that tree shrews can be infected with HBV and they successfully used this HBV-infected tree shrew model for liver cancer research. Recently Walter et al[45] reported their in vivo and in vitro study results from tree shrews infected with HBV. Yan and Li reported a significantly higher incidence of HCC in tree shrews both infected with human HBV and exposed to AFB1 than with either agent alone[46,47]. Thus, this tree shrew model appears to closely mirror the most common causative factors of human HCC in some prevalent regions. Furthermore, with the exception of the chimpanzee, tree shrew is the only known animal that can be infected with human HBV. Therefore, the application of tree shrew in research related to liver cancer and hepatitis is receiving increasing attentions and a number of works have been published[48-52].
Because of the difficulties in raising tree shrews artificially, most of the tree shrews used so far for research in China are captured individually from Yunnan Province. The drawback of using tree shrews captured in the wild for animal experiments is that their age, health status and reproductive history are unknown. In an attempt to avoid this drawback, we have conducted a preliminary experiment on rearing tree shrews and a promising result was obtained[53].
In order to study the preventive effect of oltipraz on AFB1 by animal models other than rodents, a short-term experiment was conducted on tree shrews.
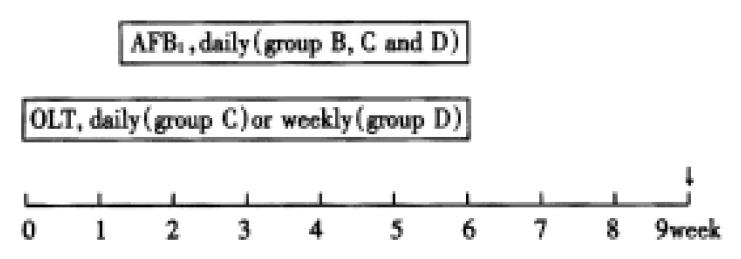
Male and female adult tree shrews (Tupaia belangeri chinensis) were purchased from the Kunming Institute of Zoology (Yunnan Province, P.R. China). Their body weights ranged from 100 g to 160 g. Upon arrival, 1 mL blood was collected from each animal and tested for HBV markers (HBsAg, anti-HBsAg, anti-HBcAg) and ALT. The tree shrews that were negative for these markers of HBV infection and that had ALT value below 55 units were divided into 4 groups with 6 or 7 animals for each group. Group A: normal control; group B: AFB1 alone; group C: AFB1 + oltipraz daily; group D: AFB1 + oltipraz weekly. All the tree shrews were allowed 1 week to acclimatize to the facilities prior to the experiment. They were housed in separate, suspended, stainless steel wire cages under controlled environmental conditions with a 12-hour light/dark photop eriod. They had free access to tap water and a natural ingredient diet.
The experimental design is presented schemati-cally in Figure 1. Tree shre ws in groups B, C and D were given AFB1 (400 μg/kg b.w./day in liquid milk) daily beginning at one week after the experiment started and continued for 4 weeks. One week before giving AFB1, tree shrews in group C and D were respectively given oltipraz (0.5 mmol/kg b.w.) daily or weekly, by gavage in a saturated solution of sucrose for 5 weeks. Blood samples and 24-hour urine samples were collected once a week from each animal throughout the experiment. At the termination of the 9-week experiment, tree shrews were killed by cervical dislocation. Three blocks of liver tissue were taken from each animal. Serial sections from each block were stained histochemically for r-glutamyl transpeptidase (γ-GT)[54] and HE respectively. The γ-GT positive liver cells were counted with a nest-ruler under microscope. The results were analyzed by the medical statistics analyzing software PEMS that was designed by West China University of Medical Sciences. The levels of aflatoxin-albumin adducts in serum samples were determined by radioimmune assay[35] and the levels of Aflatoxin-N7-guanine adducts in urine samples were assayed by HPLC[29].
No γ-GT positive liver cell focus, a postulated precancerous marker[54-56] was observed in any liver of the variously treated tree shrews in our study. However, different numbers of γ-GT positive liver cells, which scattered mainly around the portal spaces, were observed in each group. Even though the distribution patterns of these cells were similar among the 4 groups, the number was quite different. Groups B and D had obviously less γ-GT positive cells than groups A and C (Table 1).
γ-GT normally exists in embryonic liver cell in human being and rat. In adult rat, it exists only in some cells around portal spaces[57] but can be re-expressed by mature hepatocytes during the recovery process after liver damage[58]. In this study a number of γ-GT positive hepatocytes presented in periportal regions in the normal control group. On the contrary, the number of γ-GT positive hepatocytes of the same sites was markedly reduced in the AFB1 treated B group. This phenomenon is fairly consistent with the findings on AFB1 induced damage in rat, in which the periportal hepatocytes are the major targets of AFB1. As shown in the same table, the number of γ-GT positive hepatocytes in group C was strikingly similar to the normal control group. This result indicates strongly the preventive action of oltipraz against AFB1 toxicity. The apparent ineffectiveness of oltipraz in group D is most possibly due to its inadequate dose[59]. These results might indicate that oltipraz has the preventive was dose-related effect on AFB1.
Insufficient duration and/or insufficient dosage of AFB1 treatment may result in that no separate focus of γ-GT positive liver cell formed at the end of this 9-week experiment[60]. However, the decrease of γ-GT positive hepatocytes in the periportal regions may be an early marker for the damage induced by AFB1.
The levels of both aflatoxin-albumin adducts in serum samples and aflatoxin-N7-guanine adducts in urine samples of the tree shrews were also significantly affected by oltipraz. Following daily exposures to AFB1, the levels of serum aflatoxin-albumin adducts in group B increased rapidly over 2 weeks to reach a plateau that did not diminish until cessation of AFB1 exposure. In group C however, oltipraz attenuated the aflatoxin-albumin adducts significantly (P < 0.05) with a median reduction of 80%. The mean levels of aflatoxin-N7-guanine (ng/mg creatinine) in the urine samples collected at week 5 were 6.34 ± 2.04 and 0.47 ± 0.13 in groups B and C respectively. This 93% decrease represented a statistically significant difference (P < 0.05). These results were reported in detail in another article[61]. The major mechanism of oltipraz’s chemopreventive effect is probably through inducing the activities of cytochrome P450 system and phase 2 enzymes such as glutathione transferases, epoxide hydrolase, etc, as reported by Langouet et al[38]and Fahey et al[62].
Tree shrew is phylogenetically more closely related to human being than rodents. It is susceptible both to HBV infection and AFB1 intoxication. It is a suitable experimental animal for hepatocar-cinogenesis. Attempt for its rearing is promising.
Oltipraz is an effective reagent to protect AFB1 intoxication. This effect is proved clearly not only by histological examination, but also by reduction of aflatoxin-albumin adducts in serum and aflatoxin-N7 guanine adducts in urine.
All of these studies mentioned above provide a foundation for further HCC chemoprevention study by using tree shrews in the future.
The authors express their appreciation to Drs. Guo-Hua Huang, Chao Ou, Xue-Lan Deng and Hua-Ping Huang for the contributions to the animal experiment. Preliminary accounts of this work were presented at the 1999 Annual Meeting of the American Association for Cancer Research[63].
Supported in part by USPHS grant CA-39416.
Edited by You DY Verified by Ma JY
| 1. | Skolnick AA. Armed with epidemiologic research, China launches programs to prevent liver cancer. JAMA. 1996;276:1458-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Guyton KZ, Kensler TW. Prevention of liver cancer. Curr Opin Oncol. 1997;9:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Li LD, Lu FZ, Zhang SW, Mu R, Sun XD, Huangpu XM, Sun J, Zhou YS, Ouyang NH, Rao KQ. The alterations of malignant tumors'mortality in China during the 20 years and the forecasting. Zhonghua Zhongliu Zazhi. 1997;19:3-9. |
| 4. | Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 399] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Wild CP, Jiang YZ, Allen SJ, Jansen LA, Hall AJ, Montesano R. Aflatoxin-albumin adducts in human sera from different regions of the world. Carcinogenesis. 1990;11:2271-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Ruan CC, Chen YH, Zhang ZQ. Drinking water and liver cancer. China Natl J New Gastroenterol. 1997;3:47-49. |
| 7. | Deng ZL, Ma Y. Aflatoxin sufferer and p53 gene mutation in hepatocellular carcinoma. World J Gastroenterol. 1998;4:28-29. [PubMed] |
| 8. | Chen JG. The epidemeologic tendency and forecasting of liver cancer in Qidong (Abstract). Zhonghua Yufang Yixue Zazhi. 1996;30:180. |
| 9. | Groopman JD, Zhu JQ, Donahue PR, Pikul A, Zhang LS, Chen JS, Wogan GN. Molecular dosimetry of urinary aflatoxin-DNA adducts in people living in Guangxi Autonomous Region, People's Republic of China. Cancer Res. 1992;52:45-52. [PubMed] |
| 10. | Yeh FS, Yu MC, Mo CC, Luo S, Tong MJ, Henderson BE. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989;49:2506-2509. [PubMed] |
| 11. | Wogan GN. Aflatoxin as a human carcinogen. Hepatology. 1999;30:573-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Choy WN. A review of the dose-response induction of DNA adducts by aflatoxin B1 and its implications to quantitative cancer-risk assessment. Mutat Res. 1993;296:181-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Wang D, Shi JQ. Overexpression and mutations of tumor suppressor gene p53 in hepatocellular carcinoma. China Natl J New Gastroenterol. 1996;2:161-164. |
| 14. | Qian GS, Ross RK, Yu MC, Yuan JM, Gao YT, Henderson BE, Wogan GN, Groopman JD. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3:3-10. [PubMed] |
| 15. | Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 406] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Chen CJ, Wang LY, Lu SN, Wu MH, You SL, Zhang YJ, Wang LW, Santella RM. Elevated aflatoxin exposure and increased risk of hepatocellular carcinoma. Hepatology. 1996;24:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Wogan GN. Biomarkers for molecular epidemiology of aflatoxin as a risk factor for hepatocellular carcinoma: the essential role of basic science. CIIT Activities. 1999;19:4-10. |
| 18. | Wang LY, Hatch M, Chen CJ, Levin B, You SL, Lu SN, Wu MH, Wu WP, Wang LW, Wang Q. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int J Cancer. 1996;67:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Lunn RM, Zhang YJ, Wang LY, Chen CJ, Lee PH, Lee CS, Tsai WY, Santella RM. p53 mutations, chronic hepatitis B virus infection, and aflatoxin exposure in hepatocellular carcinoma in Taiwan. Cancer Res. 1997;57:3471-3477. [PubMed] |
| 20. | McGlynn KA, Rosvold EA, Lustbader ED, Hu Y, Clapper ML, Zhou T, Wild CP, Xia XL, Baffoe Bonnie A, Ofori Adjei D. Susceptibility to hepatocellular carcinoma is associated with genetic vari ation in the enzymatic detoxification of aflatoxin B1. Proc Natl Acad Sci USA. 1995;92:2384-2387. [RCA] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 155] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 550] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 22. | Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents. Toxicol Appl Pharmacol. 1999;158:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Gu GW, Zhou HG. Traditional Chinese Medicine in prevention of liver cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:80-81. |
| 24. | Zhou HG, Gu GW. Retinods preventing liver cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:82-83. |
| 25. | Tan W, Lin DX, Xiao Y, Kadlubar FF, Chen JS. Chemoprevention of 2 amino 1 methyl 6 phenylimidazo [4,5-b] pyridine induced carcinogen DNA adducts by Chinese cabbage in rats. World J Gastroentero. 1999;5:138-142. |
| 26. | Yuan JH, Zhang RP, Zhang RG, Guo LX, Wang XW, Luo D, Xie Y, Xie H. Growth-inhibiting effects of taxol on human liver cancer in vitro and in nude mice. World J Gastroenterol. 2000;6:210-215. [PubMed] |
| 27. | Chen ZY, Yan RQ, Qin GZ, Qin LL. [Effect of six edible plants on the development of AFB1-induced gamma-glutamyltranspeptidase-positive hepatocyte foci in rats]. Zhonghua Zhongliu Zazhi. 1987;9:109-111. [PubMed] |
| 28. | Li Y, Qin GZ, Qin LL, Duan XX£¬Yan RQ. A series of experimental animal study of green tea on prevention of hepatic cancer. Sichuan Zhongliu Fangzhi. 1997;10:1-4. |
| 29. | Kensler TW, Egner PA, Dolan PM, Groopman JD, Roebuck BD. Mechanism of protection against aflatoxin tumorigenicity in rats fed 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) and related 1,2-dithiol-3-thiones and 1,2-dithiol-3-ones. Cancer Res. 1987;47:4271-4277. [PubMed] |
| 30. | Roebuck BD, Liu YL, Rogers AE, Groopman JD, Kensler TW. Protection against aflatoxin B-1 induced hepatocarcinogenesis in F344 rats by 5 (2 py razinyl) 4 methyl 1,2 dithiole 3 thione (oltipraz): predictive role for short term molecular dosimetry. Cancer Res. 1991;51:5501-5506. |
| 31. | Kensler TW, Gange SJ, Egner PA, Dolan PM, Mu¢koz A, Groopman JD, Rogers AE, Roebuck BD. Predictive value of molecular dosimetry: Individual versus group effects of oltipraz on aflatoxin albumin a dducts and risk of liver cancer. Cancer Epidemiol Biomark Prevent. 1997;6:603-610. |
| 32. | Maxuitenko YY, Curphey TJ, Kensler TW, Roebuck BD. Protection against aflatoxin B-1 induced hepatic toxicity as a short term screen of cancer chemo preventive dithiolethiones. Fundament Appl Toxicol. 1996;32:250-259. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Maxuitenko YY, Libby AH, Joyner HH, Curphey TJ, MacMillan DL, Kensler TW, Roebuck BD. Identification of dithiolethiones with better chemopreventive properties than oltipraz. Carcinogenesis. 1998;19:1609-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba A, Egner PA. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J Natl Cancer Inst. 1999;91:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 213] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Wang JS, Qian GS, Zarba A, He X, Zhu YR, Zhang BC, Jacobson L, Gange SJ, Muoz A, Kensler TW. Temporal patterns of aflatoxin albumin adducts in hepatitis B surface antigen positive and antigen negative resident s of Daxin, Qidong County, People's Republic of China. Cancer Epidemiol Biomark Prevent. 1996;5:253-261. |
| 36. | Kensler TW, He X, Otieno M, Egner PA, Jacobson LP, Chen B, Wang JS, Zhu YR, Zhang BC, Wang JB. Oltipraz chemoprevention trial in Qidong, People's Republic of China: modulation of serum aflatoxin albumin adduct biomarkers. Cancer Epidemiol Biomark Prevent. 1998;7:127-134. |
| 37. | Kensler TW, Groopman JD, Sutter TR, Curphey TJ, Roebuck BD. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem Res Toxicol. 1999;12:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Langou tS, Coles B, Morel F, Becquemont L, Beaune P, Guengerich FP, Ketterer B, Guillouzo A. Inhibition of CYP1A2 and CYP3A4 by oltipraz results in reduction of aflatoxin B-1 metabolism in human hepatocytes in primary culture. Cancer Res. 1995;55:5574-5579. |
| 39. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1194] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 40. | Wang YX, Li CY, Ma SL. Charpter 1, The classification and ecolo gy of Chinese tree shrew. Biology of Chinese tree shrews. Kunming, China: Yunnan Science and T echnology Press 1991; 21-69. |
| 41. | Bearder S, Pitts RS. Chapter 36, Prosimians and tree shrews. Poole eds. The UFAW Handbook on the Care and Management of Laboratory Animals. Sixth edition. Avon, Great Britain: Bath Press 1987; 551-567. |
| 42. | Reddy JK, Svoboda DJ, Rao MS. Induction of liver tumors by aflatoxin B1 in the tree shrew (Tupaia glis), a nonhuman primate. Cancer Res. 1976;36:151-160. [PubMed] |
| 43. | Yan RQ, Su JJ, Huang DR, Gan YC, Yang C, Huang GH. Human hepatitis B virus and hepatocellular carcinoma. I. Experimental infection of tree shrews with hepatitis B virus. J Cancer Res Clin Oncol. 1996;122:283-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Yan RQ, Su JJ, Huang DR, Gan YC, Yang C, Huang GH. Human hepatitis B virus and hepatocellular carcinoma. II. Experimental induction of hepatocellular carcinoma in tree shrews exposed to hepatitis B virus and aflatoxin B1. J Cancer Res Clin Oncol. 1996;122:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Walter E, Keist R, Niederöst B, Pult I, Blum HE. Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo. Hepatology. 1996;24:1-5. [PubMed] |
| 46. | Yan RQ. [A study on primary liver cancer in tree shrews induced by human hepatitis B virus and aflatoxin B1]. Zhonghua Binglixue Zazhi. 1989;18:19-22. [PubMed] |
| 47. | Li Y, Su JJ, Qin LL, Yang C, Ban KC, Yan RQ. Synergistic effect of hepatitis B virus and aflatoxin B1 in hepatocarcinogenesis in tree shrews. Ann Acad Med Singapore. 1999;28:67-71. [PubMed] |
| 48. | Li Y, Su JJ, Yan RQ, Qin LL, Yang C, Ban KC. Duan XX, Huang GH. Expression of insulin like growth factor II (IGF-II) protein during tree shrews'hepatocarcinogenesis differently induced by AFB-1 and/or HBV. Guangxi Yike Daxue Xuebao. 1999;16:395-398. |
| 49. | Su JJ, Qin GZ, Yan RQ, Huang DR, Yang C, Huang GH, Lotlikar PD. Expression of p53 gene in hepatocellular carcinomas induced by aflatoxin B1 with or without human hepatitis B virus in tree shrews. Experiment Mole Med. 1997;29:177-182. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 50. | Su JJ, Qin GZ, Yan RQ, Huang DR, Yang C, Lotlikar PD. The expression of insulin-like growth factor II, hepatitis B virus X antigen and p21 in experimental hepatocarcinogenesis in tree shrews. Ann Acad Med Singapore. 1999;28:62-66. [PubMed] |
| 51. | Ban KC, Su JJ, Yang C, Qin LL, Li Y, Huang GH, Luo XL, Duan XX, Yan RQ. Expression of ras gene in experimental hepatocarcinogenesis in tree shrews. Chin J Cancer Res. 1999;11:23-25. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Qin LL, Su JJ, Li Y, Yang C, Ban KC, Yian RQ. Expression of IGF- II, p53, p21 and HBxAg in precancerous events of hepatocarcinogenesis induced by AFB1 and/or HBV in tree shrews. World J Gastroenterol. 2000;6:138-139. [PubMed] |
| 53. | Li Y, Baumgartner K, MacMillan D, Roebuck BD. Hand rearing of tree shrew. Dongwuxue Zazhi, in press. . |
| 54. | Li Y, Yan RQ, Qin GZ, Qin LL, Duan XX. Reliability of a short-term test for hepatocarcinogenesis induced by aflatoxin B1. IARC Sci Publ. 1991;105:431-433. [PubMed] |
| 55. | Li Y, Su JJ, Qin LL, Yang C, Luo D, Ban KC, Huang GH, Ou C, Kensler T, Roebuck B. Chemopreventive effect of oltipraz (OLT) on AFB1 induced precan cerous changes in liver of tree shrews. Aizheng. 1999;18:34-36. |
| 56. | Zhu HZ, Zhang XL, Chen YS. Expression of glutathione S-transferase placental mRNA in hepatic preneoplastic lesions in rats. World J Gastroenterol. 1998;4:38-40. [PubMed] |
| 57. | Kitagawa T, Imai F, Sato K. Re-elevation of gamma-glutamyl transpeptidase activity in periportal hepatocytes of rats with age. Gan. 1980;71:362-366. [PubMed] |
| 58. | Kitten O, Ferry N. Mature hepatocytes actively divide and express gamma-glutamyl transpeptidase after D-galactosamine liver injury. Liver. 1998;18:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Butler WH. Acute toxicity of aflatoxin B-1in rats. Br J Cancer. 1964;18:756-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 149] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Kalengayi MM, Ronchi G, Desmet VJ. Histochemistry of gamma-glutamyl transpeptidase in rat liver during aflatoxin B1-induced carcinogenesis. J Natl Cancer Inst. 1975;55:579-588. [PubMed] |
| 61. | Li Y, Su J, Qin L, Egner PA, Wang J, Groopman JD, Kensler TW, Roebuck BD. Reduction of aflatoxin B(1) adduct biomarkers by oltipraz in the tree shrew (Tupaia belangeri chinensis). Cancer Lett. 2000;154:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367-10372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 837] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 63. | Li Y, Su JJ, Qin LL, Egner PA, Wang JS, Groopman JD, Kensler TW, Roebuck BD. Modulation of aflatoxin B-1 adducts by oltipraz in the tree shrew. Proc Am Assoc Cancer Res. 1999;40:261. |