Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.454
Revised: February 3, 2000
Accepted: February 28, 2000
Published online: June 15, 2000
- Citation: Guo WJ, Zhou GD, Wu HJ, Liu YQ, Wu RG, Zhang WD. Ultrastructural localization of glutathione S-transferase-pi in human colorectal cancer cells. World J Gastroenterol 2000; 6(3): 454-455
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/454.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.454
Placental form glutathione S-transferase (GST-pi) is a group of isoenzymes with protein combining ability. Since the reports showing that GST-pi was significantly increased in proliferative hepatic nodule induced by chemical carcinogen and in well differentiated carcinoma[1], more studies have shown[2-4] that GST-pi expressed highly in neoplasm, and could be regarded as a tumor marker. GST-pi has the antimutagenesis and antitumor ability, and is related closely with the resistance of tumor to anticancer drugs. As the description about the immunoelectron microscopical localization of GST-pi in human colorectal cancer cells was not available, we have observed the ultrastructural localization of GST-pi in colorectal cancer cells, and explored the distribution of GST-pi in these cells and its relationship with colorectal carcinogenesis.
Fresh colorectal cancer tissues (n = 6) were obtained from surgical specimens, and the colorectal mucosal tissues (n = 3) more than 10 cm away from the cancer served as controls.
Reagents Monoclonal mouse anti-GST-pi antibody (Dako Company); 10 nm colloid gold labeled goat anti-mouse IgG (Institute of Basic Medicine, Academy of Military Medical Science).
Tissue processing All fresh blocks of tissue were fixed with 2.5 mL/L glutaraldehyde and 40 mL/L paraformaldehyde, routinely dehydrated and embedded in Epon 812. Ultrathin sections were placed on nickel grids, treated with 30 mL/L H2O2 (15 min), washed thoroughly with 0.05 M, pH7.4 TBS, blocked with 1% bovine serum (30 min), dropped mouse anti-GST-pi monoclonal antibody (1:20), and placed in refrigerator (4 °C) overnight. Washed with 0.05 M, pH7.4 TBS, then with 0.02 M, pH8.2 TBS, dropped 10 nm colloid gold labeled goat anti mouse IgG (1:10), stand in room temperature for 1 h, washed with TBS, and restained with uranyl acetate followed by lead citrate. The first antibody was replaced by TBS as control. These specimens were observed under electron microscope.
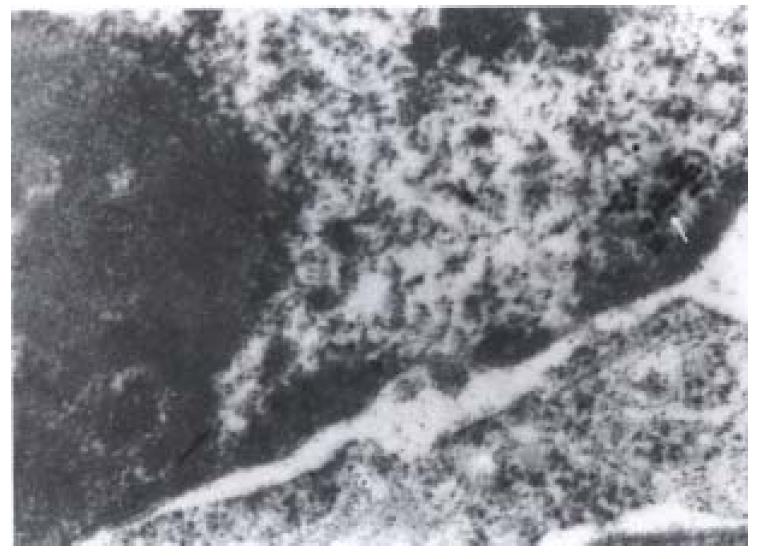
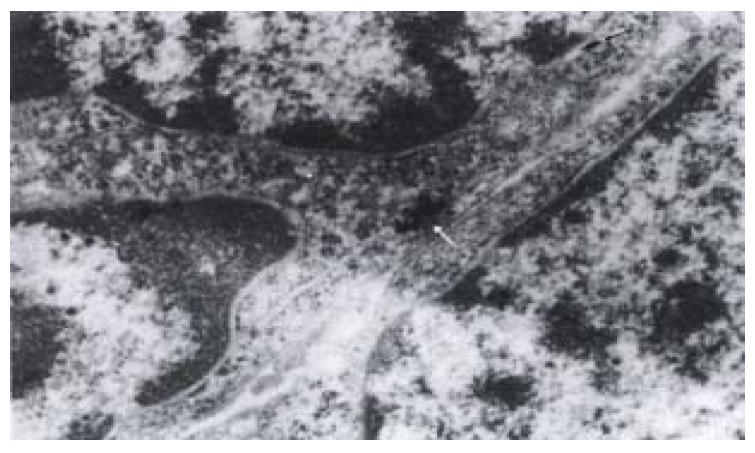
The most significant ultrastructural change of colorectal cancer cell occurred in the nucleus. It was large and irregular in shape, with peripheral aggregation of heterochromatin and plenty of ribosomes and large nucleoli. There were colloid gold labeled GST-pi positive particles in all of the six cancer specimens. The positive particles were round in shape, high and clear electron density, distributed as clusters or spots in cancer cells. The majority of positive particles were in mitochondria, lysosomes and some plasma, and some also presented in nuclei (Figure 1) or in the periphery of nuclei (Figure 2). The positive particles accumulated in lumps. Visible positive particles were never found in normal control cells.
Placental form of glutathione included rat and human forms named GST-p and GST-π respectively. Because they presented generally in placenta according to the new category, they were nominated as GST-placental isoenzyme (GST-pi) as a whole. GST-pi could detoxify chemical mutagenesis, cancer promoter, lipid and DNA hyperoxidase, and protect normal cells from the influence of cance rigenic materials, so that GST-pi played an important role in the anti-mutation and anti-cancer process. As an important enzyme of detoxification, GST-pi was controlled by the gene. T he high expression of GST-pi gene occurred while the carcinogen acted on the cells to induce their mutation[5]. The high expression of GST-pi in many neoplasms was related to the resistance of anticancer drugs. Katagiri et al[6] reported that the expression of GST-pi negatively correlated to the sensetivity of anticancer drug in testicular tumor. Dong J suggested that the expression of GST-pi closely related to resistance of anticancer drug in ovariogenic neoplasm[7]. GST-pi could be regarded as a marker in evaluating the effect of tumorectomy, or in predicting the drug resistance of tumor cells. High expression of GST-pi in precancer and cancer of liver, and the protein synthesis ability could be blocked by cyclohexamide, which showed that GST-pi was controlled in transcription level. This may be the molecular base of the cancer cells in getting cytotoxic drug resistance. We found that GST-pi expressed only in cancer cells, but not in normal cells, suggesting that this might be related to the action of carcinogen. GST-pi specific expression in colorectal cancer cells may be used as a marker for diagnosis of colorectal carcinoma.
We found that GST-pi was located in cytoplasm, mitochondria, lysosomes and nucleus adjacent to nuclear membrane of colorectal cancer cells. These agreed with immunohistochemical studies[8]. But normal mucosa occasionally showed slight positivity in immunohistochemical studies. This is different from our findings. It might be related to the weak expression in normal mucosa. There were few studies about the GST-pi ultrastractural localization. In our study, GST-pi positive particles were found not only in cytoplasm, but also in nucleus. Chen M reported the same result in bladder carcinoma[9]. Why was GST-pi positive particles present in nucleus? We think this can be an atavism, and if so it could not be used as a marker indicating colorectal cancer. Whether it is a right concept or not needs further studies.
Dr. Wen-Jun Guo, graduated from Weifang Medical College in 1979, now associate professor of pathology, majoring gastrointestinal tumor immunopathology and having more than 30 papers published.
Edited by You DY and Ma JY
proofread by Sun SM
| 1. | Sato K, Kitahara A, Satoh K, Ishikawa T, Tatematsu M, Ito N. The placental form of glutathione S-transferase as a new marker protein for preneoplasia in rat chemical hepatocarcinogenesis. Gan. 1984;75:199-202. [PubMed] |
| 2. | Tsuchida S, Sekine Y, Shineha R, Nishihira T, Sato K. Elevation of the placental glutathione S-transferase form (GST-pi) in tumor tissues and the levels in sera of patients with cancer. Cancer Res. 1989;49:5225-5229. [PubMed] |
| 3. | Cairns J, Wright C, Cattan AR, Hall AG, Cantwell BJ, Harris AL, Horne CH. Immunohistochemical demonstration of glutathione S-transferases in primary human breast carcinomas. J Pathol. 1992;166:19-25. [PubMed] |
| 4. | Hamada S, Kamada M, Furumoto H, Hirao T, Aono T. Expression of glutathione S-transferase-pi in human ovarian cancer as an indicator of resistance to chemotherapy. Gynecol Oncol. 1994;52:313-319. [PubMed] |
| 5. | Liu YB, Chen YJ, Yin Y, Yang XJ, Yang YJ, Wu DZ. The glutathione-S-transferase activity and the gene expression of GST-π in 56 tumor patients. Aizheng. 1995;14:1. |
| 6. | Katagiri A, Tomita Y, Nishiyama T, Kimura M, Sato S. Immunohistochemical detection of P-glycoprotein and GSTP1-1 in testis cancer. Br J Cancer. 1993;68:125-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Huang J, Gu M, Chen C. [Expression of glutathione S-transferase-pi in operative specimens as marker of chemoresistance in patients with ovarian cancer]. Zhonghua Fuchanke Zazhi. 1997;32:458-461. [PubMed] |
| 8. | Guo WJ, Wu HJ, Liu YQ, Zhang WD, Guo AH, Huang WB. The value of GST-pi in the early diagnosis of colon-rectal carcinoma. Weifang Yixueyuan Xuebao. 1999;21:3. |
| 9. | Chen M, Sui YG, You GC, Xu ZS, Feng SZ. Localization of glutathione S-transferase π in human bladder neoplasmas. Zhonghua Miniao Waike Zazhi. 1998;19:391. |