Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.421
Revised: February 3, 2000
Accepted: March 16, 2000
Published online: June 15, 2000
- Citation: Wang QG, He LY, Chen YW, Hu SL. Enzymohistochemical study on burn effect on rat intestinal NOS. World J Gastroenterol 2000; 6(3): 421-423
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/421.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.421
The blood irrigate flow obstruction, especially the gastrointestinal (GI) ischemia[1], is the main factor of the damage to the digestive tract caused by serious burns. The effect of GI ischemia on the whole body is extensive and profound, which not only causes the increase of intestinal permeability and the movement of bacteria and toxin in the intestinal cavity, but releases a large quantity of inflammatory media. Neuroendocrine element after burns is closely related to intestinal damage[2]. As is known at present, abundant nitric oxide synthase (NOS) is distributed in GI tract, whose product NO is a nonadrenergic and noncholinergic (NANC) restraining transmitter in enteric nervous system which participates extensively in various physiological functions in the intestinal tract[3]. Few reports about the effects on intestinal NOS after serious burns are available. We used scalded rat model with degree III 40% of body surface area (TBSA), and enzymatic histological and biochemical methods to observe dynamically the active changes of empty myenteric plexus NOS and changes of jejunal tissue MDA SOD and GSH-PX and probe into the relationship between NOS and intestinal tissue and function damage as well as their mechanism so as to provide morphological experimental basis for clinical treatment.
B-NADPH, TYPE, nitroblue telrazolium (NBT) (Sigma); SOD reagent kit (Wuhan Xiehe Hospital Science and Technology Development Centre); thibarbiluric acid (Shanghai Reagent Factory); self-made phosphate buffer; and healthy SD rats weighing 250 g provided by the Animal Laboratory Centre of Tongji Medical University.
Eighty rats were randomly divided into five groups: sham-burn control (SBC) and 4 groups of 8, 24, 48 and 72 h post burn, each group having 16 rats (40 were measured for biochemical indexes, and other 40 for morphological indexes). The rats, before being scalded, had their hair depilated on the neck and back with 80 g/L Na2S under the anaesthesia with 100 g/L trichloroacetaldehy-demonohydrate after they became conscious, depilated parts were soaked into the 100 °C boiling water for 16 sec and scalded to degree III 400 g/L TBSA. They were then fed in different cages without treatment. And those in sham-burn control group were soaked into 37 °C water.
Rats in different groups, after being scalded, were sacrificed at different time points, a 10 cm empty intestine was taken out and made into thick liquid. SOD activity was determined with modified pyrogallol auto-oxidation[4]. GSH-PX activity by active DTNB[5], and the content of MDA with modified thiobarbituric acid fluorescence analysis[6].
The rats in each group were anaesthetized with 100 g/L trichloroacetaldehy demonohydrate (300 mg/kg), opened up to expose the heart, the blood was quickly rinsed away with 200 mL warm normal saline from left chamber through ascending aorta, and then 450 mL of 40 mL/L cold parafor-maldehyde was instilled for one hour, a 10 cm empty intestine was taken out, the intestinal content was washed off and filled again with the same liquid to make the intestinal track full, both ends were ligated and fixed in the above-mentioned liquid (4 °C) for 4 h. The outer longitudinal tunica muscularis was peeled off care fully from the ring tunica muscularis and telasubmucosa, and the specimens were made.
The specimens after being washed with 0.1 mol/L of PBS, was put into reduction type NADPH-d hatching liquid at 37 °C for 50 min (cosisting of 0.1 mol/L of PB (pH8.0) which contained 30 g/L tritonx-100, 100 mol/L of nitroblue telbrazolium and 1 g/L of NADPH-d, and washed thoroughly with 0.1 mol/L of PBS. After reaction[7], it was pasted onto agalative glass piece and conventionally dehydrated, made into transparency, sealed and observed under microscope. The staining result of NOS was achieved using IBAS automatic picture analyzer to determine semi-quantitatively the contents in myenteric plexus neuron NOS and internode bind NOS.
The data were expressed as -x±s, and t test was used to compare the results.
The content of MDA in the empty intestinal tissues gradually rose with the time of burns; compared with the SBC, the contents in each group increased significantly (P < 0.01). But the activity of SOD and GSH-PX decreased markedly the difference being significant as compared with SBC (P < 0.01).
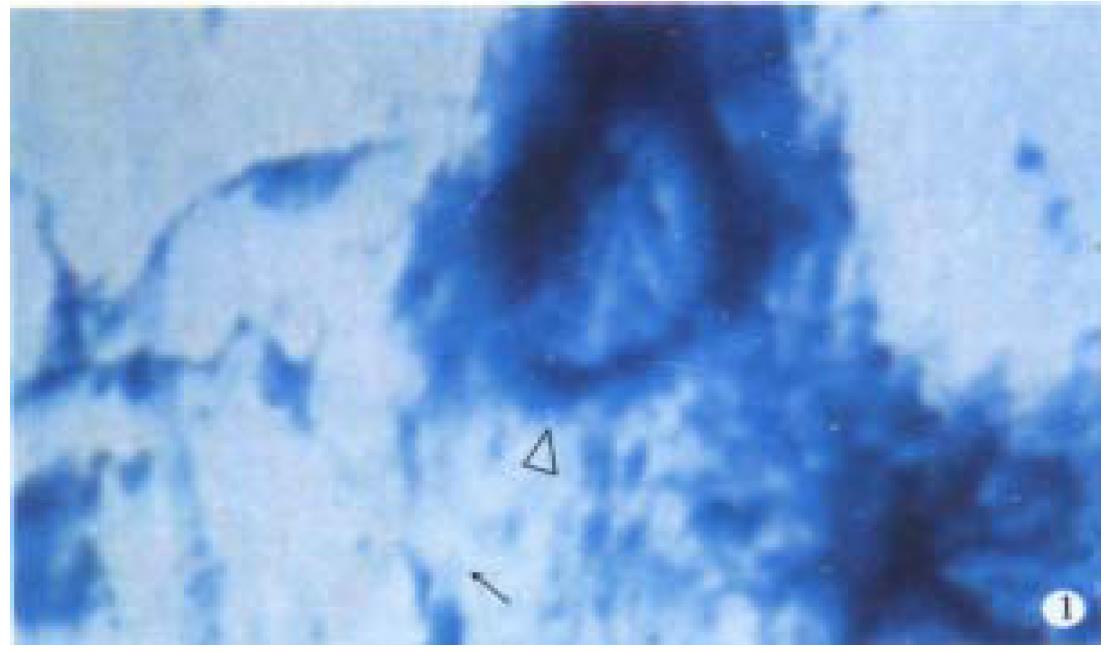
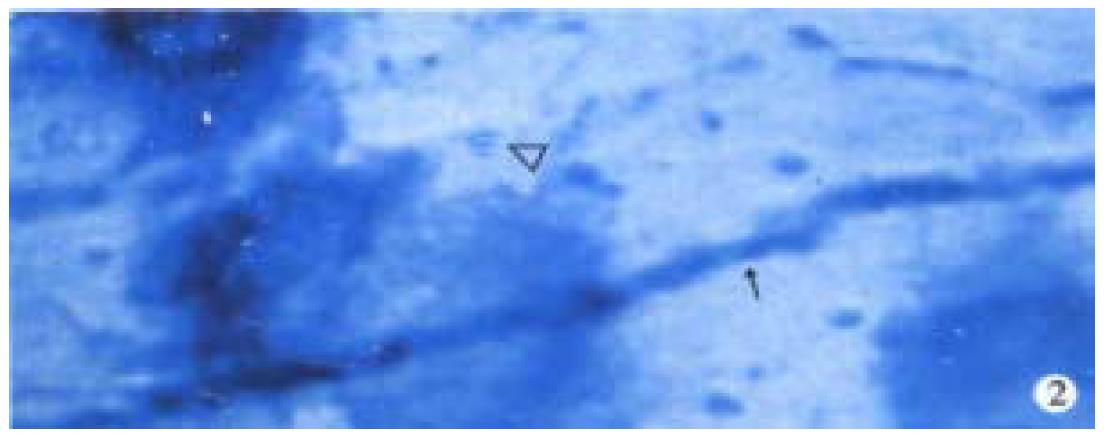
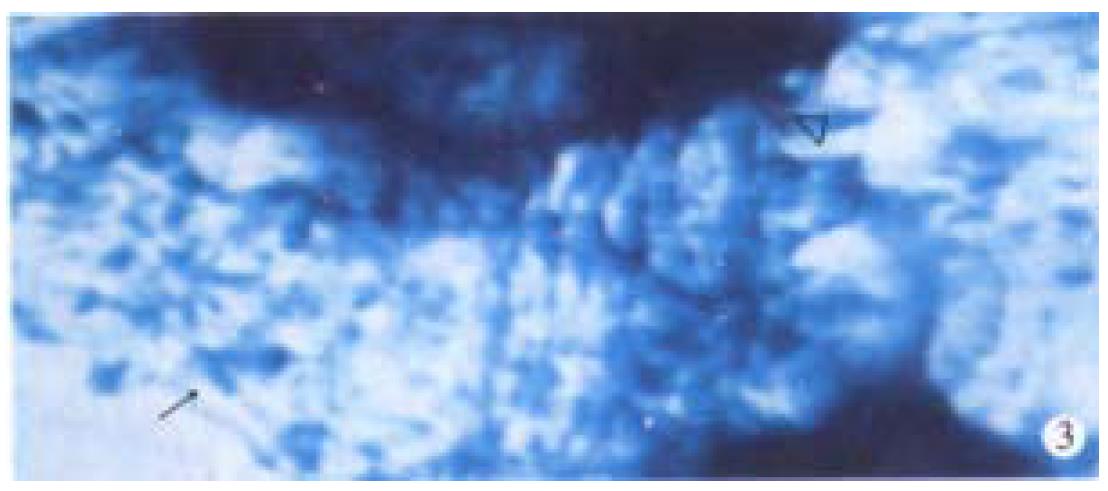
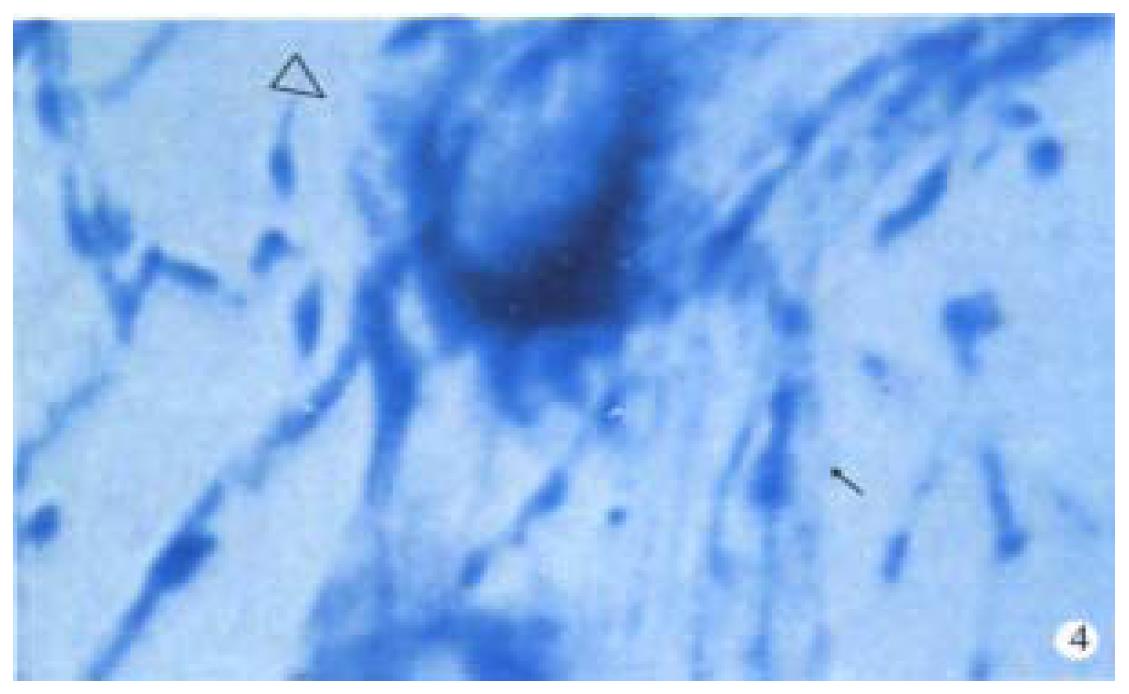
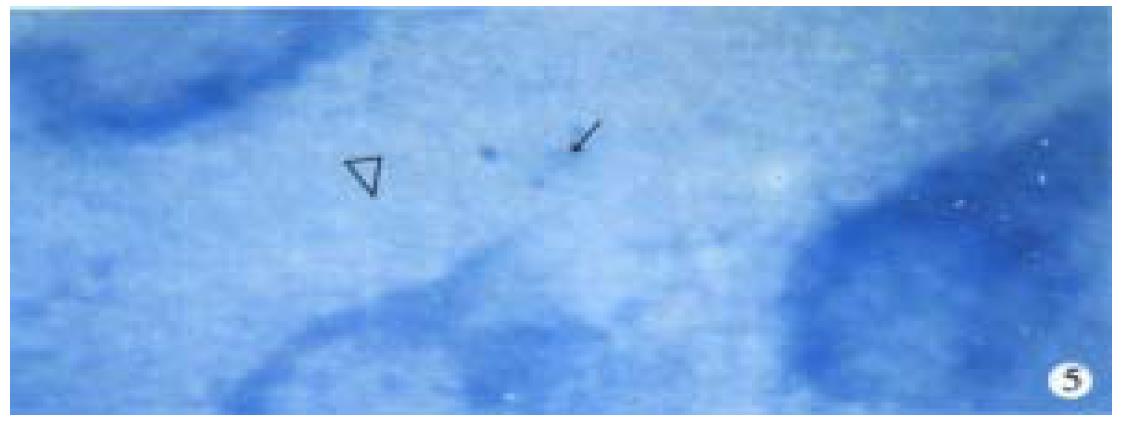
Observation under light microscope In the control group, most ganglion cells in myenteric plexus and their scabrosity appeared strongly positive in NOS, while a small number had moderate staining, most being distributed around myenteric plexus and the cell being comparatively large and various in shape, i.e., ovate, triangle or irregular. There were NOS positive products in dark blue in the cytoplasm and the nucleus was negative. Most of intersegment bind fiber contained bulge, and some had division and intersect with varying size (Figure 1). In the group of 8 h post-burn, the cell NOS stain appeared obviously lighter but intersegment bind fiber had no obvious change (Figure 2). In the 24 h post-burn group, ganglion cell NOS staining was intensified obviously with clear cell outline and larger size. Intersegment bind fiber and bulge became markedly dense (Figure 3). In the 48 to 72 h post-burn groups, ganglion cell and intersegment bind fiber staining gradually became lighter, especially in those of 72 h group (Figure 4), perikaryon and OD change of myenteric plexus NOS in the empty intestine of burned rats. In burned rats after 8 h the OD of myenteric plexus and intersegment bind fiber NOS were significantly lower than that in the control group (P < 0.01) and in those after 24 h, the OD of ganglion cells and intersegment bind fiber NOS was significantly higher than that in the control (P < 0.01); while in those after 48 to 72 h, OD of ganglion cells and intersegment bind fiber NOS decreased gradually, being significantly different from the control group and those of 24 h post-burn (P < 0.01) (Figure 5).
NO, a newly discovered active biosubstance in recent years, is a dual-functional messenger and venenosity molecule with short half-life and instable nature. The in vivo NO, in the form of L-Arg as primer, exists through the catalyzation of NOS, which indicates that the distribution of NOS is closely related to the physiological functions of NO. It is reasonable to infer bioeffects of NO through the research of NOS, Nathan et al[8] divided NOS into two types, i.e. cNOS and iNOS. cNOS is mainly distributed in neurocyte and endothelium and catalyzes NO which acts chiefly as neurotransmitter and secondary messenger, while iNOS, mainly distributed in macrophage and endothelium cell, catalyzes NO, which has cytotoxic effect. However, the physiological effect of NO to the body depends chiefly on the quality and strength of stimulative elements, the dosage and reactive sites. Thus we can presume that after serious burns bad perfusion of intestinal blood, disorder of motor functions and damaged enteromycoderm may be related to the abnormal intestinal NOS activity. It was suggested that MDA content in the jejunal tissues of the burned rats increased, the result was consistent with that of Peng et al[9]. At the same time, SOD and GSH-PX antioxidase activities in jejunal tissues decreased after being burned, indicating that lipoperoxidation reactions participate in the pathophysiological course of the mucosal damage of burned rats. Some studies demonstrated that cNOS was distributed extensively in plexuses of GI wall, and its output NO was inhibitory neurotransmitter of NANC nerves in GI tract, which may cause angiectasis of intestinal tract and laxation of smooth muscles[10]. Eight hours after serious burns myenteric plexus NOS activity decreased obviously in our experiment suggesting burn stress caused adrenergic nerve excitation, and inhibited NANC nerves, leading to the decrease of cNOS activity and the catalyzed NO, causing excessive contraction of smooth muscles. These may be the main causes in bad perfusion of intestinal blood and disorder of motor functions. In this experiment, 24 h post-burn, myenteric plexus NOS activity increased significantly, possibly because the enterogenous infection after serious burn, activated the endotoxin on macrophage cell of intestine to release a series of such body fluid agents as TNT IL-1, etc, leading to increased iNOS activity. A large amount of high-concentration NO produced in this way had cytotoxic effects which further damaged the intestinal tissues. Excessive amounts of NO and O-2 free radicals produced more toxic ONOO - free radicals which further react to produce HO-, NO2, NO-3, etc. while HO- and NO2 are catalyst of biomembrane lipid peroxidation and can cause a succession of peroxidation of high unsaturation and adipoacid occurring on the membrane, thus producing a large amount of metabolin MDA worsening the damage of intestinal tissues. Therefore 48-72 h post-burn, we found that intestinal myenteric plexus ganglion cell and perikaryon reduced obviously, and changes caused by the lowered NOS activity and other delayed neuronal damage. It can be inferred that NO in the intestine of seriously burned rats and ONOO- reacted by O-2 are important mechanisms that NO damages intestinal structure and functions. It suggests that the damaged structure and functions in seriously burned rats are related to the increase of NOS activity. It is concluded that using the inhibitor of NOS to lower NOS activity level may be beneficial to lessening the degree of post-burn intestinal damage.
Edited by Ma JY
| 1. | Cui X, Sheng Z, Guo Z. [Mechanisms of early gastro-intestinal ischemia after burn: hemodynamic and hemorrheologic features]. Zhonghua Zhengxing Shaoshang Waike Zazhi. 1998;14:262-265. [PubMed] |
| 2. | Hu D, Chen B, Lin S. [Observation and analysis of postburn changes in substance P (SP) and SP peptidergic nerve fibers in the intestine of rat]. Zhonghua Zhengxing Shaoshang Waike Zazhi. 1996;12:93-97. [PubMed] |
| 3. | Rand MJ. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992;19:147-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 284] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Deng BY, Yuan QS, Li WJ. Superoxide dismruase assayed by modified pyrogallolaut oxidation mothod. Progress Biochem Biophysics. 1991;18:163. |
| 5. | Xia YM, Zhu LZ. The datermination of glutathione peroxidase in blood and tissue-DTNB direct method. Weisheng Yanjiu. 1987;16:29-33. |
| 6. | Zhang XM, Yan LJ, Chai JK, Zhou WQ, Wang LX. Improvement of the thiobarbituric acid fluorescence analysis of serum lipoperoxides. Progress Biochem Biophysics. 1996;23:175-179. |
| 7. | Ding YQ, Lou XF, Wang YQ, Qin BZ. The distribution of nitric oxide synthase positive neurons and fibers and the origin of the positive fibers in the rat spinal cord. Chin J Neuroanat. 1993;9:81-87. |
| 8. | Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2132] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 9. | Peng YZ, Xiao GX, Wang DW, Zhang YP, Qin XJ. Role of lipid peroxidation in the mechanism of bacterial translocation from the gut after severe thermal injury in rats. Disan Junyi Daxue Xuebao. 1989;11:329-334. |
| 10. | Nichols K, Krantis A, Staines W. Histochemical localization of nitric oxide-synthesizing neurons and vascular sites in the guinea-pig intestine. Neuroscience. 1992;51:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |