Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.402
Revised: February 3, 2000
Accepted: February 18, 2000
Published online: June 15, 2000
- Citation: Hu YY, Liu CH, Wang RP, Liu C, Zhu DY, Liu P. Protective actions of salvianolic acid A on hepatocyte injured by peroxidation in vitro. World J Gastroenterol 2000; 6(3): 402-404
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/402.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.402
Salvianolic radix, one of the most commonly used traditional Chinese herbs, was widely studied about its actions against liver injury and fibrosis, and was one of the focuses of recent research[1,2]. Salvianolic acid-A (SA-A) was an aqueous soluble component of Salvianolic radix. In our previous work[2], SA-A was found to have protective effects against liver injury and fibrosis induced by carbon tetrachloride (CCl4) in rats. In order to investigate the effect of SA-A on peroxidation in hepatocytes, we induced the injured hepatocyte model by CCl4 fumigation in vitro, treated the cell model with SA-A or aqueous soluble vitamin E (Vitamin E), the latter served as the control drug, and observed the influences of the drugs on the functions of the hepatocytes injured by peroxidation.
Wistar rats, male, specific pathogens free (SPF), weighing 140-160 g, were provided by the Experimental Center of Animals, Shanghai University of Traditional Chinese Medicine.
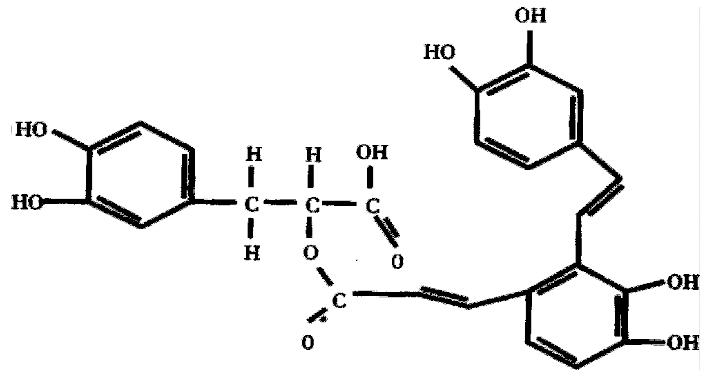
SA-A, molecular formula C26H22O10, molecular structure as Figure 1, weight 494, was extracted and identified by Shanghai Institute of Materia Medica, Chinese Academy of Sciences. Vitamin E was purchased from Hoffman Co. USA.
Purified type III collagenase (specific activity, 960 U/mg), insulin and dexamethasone were purchased from Sigma Co. USA. Soybean trypsin was from Institute of Biochemistry, Chinese Academy of Science; ficoll was from Shanghai Second Chemical Reagent Factory; medium 199 (M199) from Gibco Co., USA; carbon tetrachloride (CCl4), analytical grade, from Yixin Third Chemical Reagent Factory, Jiangsu Province; and newborn bovine serum (NBS) from Shanghai Sino-American Co.
According to a modified method[3], hepatocytes were isolated and primarily cultured from rats. In brief, after anesthesia with ether, the rat liver was perfused in situ with Ca2+ and Mg2+ - free Hank's solution via the portal vein for 5 min, followed by perfusion with Hank's solution containing 0.5 g/L collagenase for 20 min. The liver was then excised and minced with forceps to remove Glisson's capsule and the liver cells dispersed. The liver cell suspension was filtrated with double layers of gauze and was adjusted to 2 × 1010/L. Then 5 mL cell suspension was added onto the top of 20 mL 492 g/L Ficoll, and centrifuged at 50 ×g, 4 °C, 5 min to purify the hepatocytes. The cell recovery was about 1 × 1010 cells per liver, the purity was more than 95% identified by the cell typical appearance via phase contrast microscope, and the viability was more than 90% assessed by trypan blue exclusion. Hepatocytes were suspended with M199 containing 5% (v/v) NBS to adjust their density to 5 × 108 cells/L, seeded on plastic dishes (Nunc, Denmark) and primarily cultured at 37 °C in a humidified atmosphere of 50 mL/L CO2 and 950 mL/L air.
According to David method[4], the peroxidatic injury of hepatocytes was induced by CCl4 fumigation. Briefly, after 48 h of isolation and culture, the cells were placed in sealed box, to which 1 mL/L CCl4 was added, and the cells were fumigated with CCl4 at 37 °C for 24 h.
Normal hepatocytes in dishes were divided into the following groups: the normal, the control, vitamin E (2 × 10-3 mol/L[5]) and SA-A treated groups at different concentrations (10-4-10-8 mol/L). All but the normal group, were incubated with vitamin E or SA-A at different concentrations and fumigated with CCl4 spontaneously for 24 h, then the culture medium was collected respectively and stored at -70 °C until assay.
The ALT and AST in culture medium were assayed with Rriman and Frankle method, malondiadehyde (MDA) with Bacon's method[6]. Superoxide dismutase (SOD), catalase (CAT), lactase dehydrogenase (LDH), glutathione peroxidase (GSH-PX), glutathione (GSH) were measured following the protocols provided by the manufacturer (Jianchueng Biochemical Technological Institute, Nanjing).
Chi- square test and q test.
After 48 h of isolation and culture, primary hepatocytes gathered, attached and grew very well. At 24 h after fumigation with CCl4, the hepatocytes partially shrank, their plasma membrane became rough and organelles swollen. When the cell models were incubated with SA-A or Vitamin E, the plasma membrane became smoother and the organelles less smother than those of controlled model cells.
The activities of ALT and AST both increased, while the former increase more obviously. LDH activity enhanced approximately 20 folds. SA-A inhibited these pathological increase in dosage dependent manner, and among all concentrations tested the 10-4 mol/L SA-A had the best effect on the cell structure and enzymes. The effect of SA-A was better than that of Vitamin E, but 10-7 mol/L-10-8 mol/L SA-A was not effective compared with the control (Table 1).
| Group | ALT (U·L-1) | AST (U·L-1) | LDH (U·L-1) |
| Normal | 18 ± 2.8b | 56 ± 3.8b | 77 ± 38b |
| Control | 103 ± 6.5 | 176 ± 9.1 | 1674 ± 128 |
| 10-4 mol·L-1 SA-A | 49 ± 2.9bd | 134 ± 5.0bd | 1050 ± 83bd |
| 10-5 mol/L SA-A | 72 ± 3.9bd | 177 ± 8.3 | 1551 ± 88 |
| 10-6 mol/L SA-A | 91 ± 11.1a | 177 ± 9.2 | 1602 ± 88 |
| 10-7 mol/L SA-A | 96 ± 7.9 | 181 ± 6.7 | 1657 ± 81 |
| 10-8 mol/L SA-A | 93 ± 11.4 | 181 ± 10.7 | 1684 ± 71 |
| Vitamin E | 86 ± 7.6b | 182 ± 10.7 | 1509 ± 30a |
MDA content in the control nearly doubled that of the normal, and the activities of SOD and CAT increased remarkably. SA-A decreased these pathological changes, and 10-4 mol/L SA-A had a significant inhibitory action. Vitamin E also decreased the MDA content markedly, but had no obvious influence on SOD activity (Table 2).
| Group | MDA (μmol·L-1) | SOD (U·L-1) | CAT (U·L-1) | GSH (U·L-1) | GSH-PX (U·L-1) |
| Normal | 5.11 ± 0.91b | 30.4 ± 2.86b | 12.8 ± 3.45b | 1.27 ± 0.13b | 16.7 ± 8.84b |
| Control | 9.17 ± 0.80 | 59.0 ± 2.23 | 86.6 ± 13.00 | 0.36 ± 0.07 | 90.9 ± 11.00 |
| 10-3 mol/L SA-A | 6.79 ± 0.81a | 45.6 ± 3.26bd | 17.3 ± 3.59bd | 0.95 ± 0.02bd | 65.2 ± 1.24a |
| 10-4 mol/L SA-A | 7.67 ± 1.11 | 55.2 ± 2.44 | 38.3 ± 11.82b | 0.40 ± 0.04 | 81.8 ± 17.54 |
| 10-5 mol/L SA-A | 8.27 ± 1.50 | 57.6 ± 3.27 | 49.9 ± 6.78b | 0.38 ± 0.10 | 84.1 ± 19.11 |
| Vitamin E | 7.52 ± 0.69a | 55.8 ± 4.03 | 27.6 ± 3.22b | 0.49 ± 0.07a | 63.64 ± 10.57a |
After the hepatocytes were injured by peroxidation, the GSH-PX activity increased but GSH content decreased remarkably. 10-4 mol/L SA-A or Vitamin E inhibited the increase of GSH-PX activity and the decrease of GSH. For the extent of inhibition in GSH lowering, SA-A 10-4 mol/L is superior to Vitamin E (Table 2).
In this study, 24 h after fumigation of hepatocytes with CCl4, the ALT, AST and LDH all increased remarkably, the rate of elevation was in order of LDH, ALT and AST. It is suggested that the hepatocytes were acutely injured, cell membrane integrity was broken and the enzymes in cell plasma leaked out. However, after the hepatocytes injured by peroxidation which were incubated with SA-A, the pathological increases of ALT, AST and LDH reduced markedly. It is indicated that SA-A had a potential effect against hepatocyte injury.
The free radicals and its triggered lipid peroxidation were involved in the main mechanisms by which carbon tetrachloride injured hepatocytes. MDA was one of main lipid peroxidatic products, its elevated levels could reflect the degrees of lipid peroxidatic injury in hepatocytes. GSH, a peroxide scavenger with a lower molecular weight, could eliminate superoxide anion and hydrogen peroxide. The content of GSH reflected the ability against peroxidation[7]. In this study, GSH in hepatocytes of the model group was reduced remarkably, suggesting that the potency of antioxidation in injured cells was decreased. There were many other markers that could reflect lipid peroxidation, e.g. SOD, a scavenger of peroxide anion radicals, which could inhibit the initiation of lipid peroxidation by free radicals; GHS-PX, which could particularly catalyze the reductive action of GSH to H2O2 to protect the integrity of plasma membrane and functions; CAT etc. All the above-mentioned enzymes increased in the model cells. This may result from acute compensation after injury, and peroxidatic reaction stimulated by CCl4 in hepatocytes. SA-A markedly inhibits the increase of MDA level and the decrease of GSH, also reduced the activities of GSH-PX, CAT, SOD in different extents. Among these results, SA-A had better effect than vitamin E, which is a widely recognized antioxidation drug. It is indicated that SA-A had potential action against lipid peroxidation, this effect perhaps is the main mechanism of protection on liver injury. The results are also in accordance with the other reports[8] and our previous work[2].
The project was supported by Shanghai Education Committee "Shuguang Program", NO. 96 SG 26.
Edited by You DY
proofread by Sun SM
| 1. | Deng H, Ma X, Xu R, Chen X, Zhao Y, Yin L, Han D. [Mechanisms of protective action of radix Salviae miltiorrhizae (RSM) against experimental hepatic injury in rats]. Zhongguo Zhongyao Zazhi. 1992;17:233-26, inside backcover. [PubMed] |
| 2. | Hu YY, Liu P, Liu C, Xu LM, Liu CH, Zhu DY, Huang MF. [Actions of salvianolic acid A on CCl4-poisoned liver injury and fibrosis in rats]. Zhongguo Yaoli Xuebao. 1997;18:478-480. [PubMed] |
| 3. | Alpini G, Phillips JO, Vroman B, LaRusso NF. Recent advances in the isolation of liver cells. Hepatology. 1994;20:494-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Johnston DE, Kroening C. Stimulation of prostaglandin synthesis in cultured liver cells by CCl4. Hepatology. 1996;24:677-684. [PubMed] |
| 5. | Zhu JL, Liu SL, Wu J, Tsyganskaya M, Kuncio GS, Zern MA. MG132, vitamin E and lithospermic acid A all inhibit TGHP induced damage to rat hepatocytes. Hepatology. 1996;24:335A. |
| 6. | Bacon BR, Tavill AS, Brittenham GM, Park CH, Recknagel RO. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest. 1983;71:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 289] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Gassó M, Rubio M, Varela G, Cabré M, Caballería J, Alonso E, Deulofem R, Camps J, Giménez A, Pajares M. Effects of S-adenosylmethionine on lipid peroxidation and liver fibrogenesis in carbon tetrachloride-induced cirrhosis. J Hepatol. 1996;25:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Lin TJ, Liu GT. Protective effect of salvianolic acid A on heart and liver mitochondria injury induced by oxygen radicals in rats. Zhongguo Yaolixue Yu Dulixue Zazhi. 1991;5:276-281. |