Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.365
Revised: February 3, 2000
Accepted: March 2, 2000
Published online: June 15, 2000
AIM: To develop a culture mode providing durable biomaterials with high yields and activities used in bioartificial liver.
METHODS: Hepatocytes were isolated from a whole pig liver by Seglen′s method of orthotopic perfusion with collagenase. In culture on microcarriers, primary porcine hepatocytes were inoculated at a concentration of 5 × 107/mL into the static culture systems containing 2 g/L Cytodex-3, then supplemented with 100 mL/L fetal calf serum (FCS) or 100 mL/L porcine portal vein serum (PPVS) respectively. In spheroidal aggregate culture hepatocytes were inoculated into 100 mL siliconized flasks at a concentrati on of 5.0 × 106/mL.
RESULTS: In culture on microcarriers hepatocytes tended to aggregate on Cytodex-3 obviously after being inoculated. Typical multi-cellular aggregated spheroids could be found in the two systems 24-48 h after hepatocytes were cultured. The morphological charact-eristics and synthetic functions were maintained for 5 wk in FCS culture system and 8 wk in PPVS culture system. In spheroidal aggregate culture about 80%-90% isolated hepatocytes became aggregated spheroids 24 h after cultured in suspension and mean diameter of the spheroids was 100 μm. The relationship among the hepatocytes resembled that in the liver in vivo. Synthetic functions of albumin and urea of the spheroids were twice those of hepatocytes cultured on monolayers.
CONCLUSION: As high-yields and high-activity modes of culture on microcarriers or in spheroidal aggregate culture with portal vein serum are promising to provide biomaterials for bioartificial liver (BAL) efficiently.
- Citation: Gao Y, Hu HZ, Yang JZ, Chen K. Primary porcine hepatocytes with portal vein serum cultured on microcarriers or in spheroidal aggregates. World J Gastroenterol 2000; 6(3): 365-370
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/365.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.365
Great attention was paid world-wide to the bioartificial liver (BAL) aiming to release the clinical symptoms of severe acute liver diseases or liver injuries and act as a transitional bridge for liver transplantation. Modern BAL is mainly characterized by its multiple biofunctions resembling those of liver in vivo and such functions may be completely derived from the biomaterials in BAL. Years of researches at home and abroad showed that primary hepatocytes were the most ideal biomaterial of BAL. But for the morphological and functional changes of primary hepatocytes cultivated in vitro occurred obviously in short time, it was deadly restrained to use them as the biomaterial in BAL. To probe into this question, together with our proceeding researches on this subject[1], we took the method of two-step orthotopic collagenase perfusion to isolate hepatocytes and cultured them on microcarriers Cytodex-3 statically in flasks in the systems supplemented with FCS or PPVS respectively, and also cultured them in the matrix DMEM containing porcine portal vein serum at a concentration of 100 mL/L through the way of spheroidal aggregating, then estimated the possibility of such hepatocytes acting as the biomaterial in BAL in view of their morphology and-biofunctions.
The experimental animals were five native sex-unlimited pigs aged 1-2 mo and weighed 10-15 kg from the experiment animal center of SUN Yat-Sen Medical University. Insulin, glucagon, transferrin, collagenase IV, typran and microcarrier Cytodex-3 were perchased from Sigma (USA). Solution of 50 g/L methyl silicon resin ethyl acetate was confected by ourselves. Ten g/L osmic acid, 20 g/L pentanal and gradient acetone solutions were provided by the Department of Electron microscope of First Military Medical University. S-450 electron scanning microscope was from Japan. PPVS was collected in the experiment. The liver perfusion solution was prepared according to Table 1.
| NaCl | KCl | NaOH | CaCl2 | MgCl2 | Na2SO4 | HEPES | Collagen ase (IV) | Tricine | Tes | pH | |
| I | 8.3 | 0.5 | 0.22 | 2.4 | 7.4 | ||||||
| II | 3.9 | 0.5 | 2.6 | 0.53 | 24 | 0.5 | 7.4-7.6 | ||||
| III | 4.0 | 0.4 | 2.1 | 0.136 | 0.13 | 0.1 | 7.2 | 6.5 | 6.9 | 7.4 | |
| IV | 8.3 | 0.5 | 0.11 | 0.136 | 2.4 | 7.4 |
Isolation of porcine hepatocytes The experimenal pig was kept fasting for 12 h preoperatively and anaesthetized with 30 g/L sodium barbiturate ip. After the abdominal skin being prepared and disinfected, celiotomy was performed, then portal vein and inferior caval vein below the liver exposed and 0.25 mg (125 IU) heparin injected into the inferior caval vein. After being dissected, the portal vein and the inferior caval vein were ligated at the distal end and intubated at the proximal end respectively. When the inferior caval vein above the liver ligated at the superior end of liver vein, successive perfusing solutions I and II pre-warmed in 37 °C water bath were perfused along the circuit via portal vein to inferior caval vein. Perfusing speed was about 50-60 mL/min for 20 min. Perfusion was ended until the liver became pallor or yellow in color. Then the well digested liver were taken out, tore off the liver sheath and the isolated hepatocytes delivered into solution III pre-warmed in 37 °C water bath. After being filtered by 150 μm, 100 μm and 80 μm sieve, the hepatocytes were suspended in solution and centrifuged at a speed of 1000 r/min for 3-5 min repeated three times. The deposited hepatocytes were added into solution IV to prepare the hepatocyte suspension for use. Portal vein blood was gathered from the distal end of the ligated portal vein and in a total amount of 1300 mL from 5 pigs. It was centrifuged at a 2000 r/min to isolate the portal vein serum, and the very serum centrifuged three times more to prepare the refined serum which finally amounted to 714 mL. The refined serum was filtered to be excluded from bacteria and preserved separately for use. Sample from the suspension was observed under light microscope to calculate the yielding rate of hepatocytes and stained by typran for determination of their activity.
Static culture of hepatocytes on microcarriers in flasks Porcine hepatocyte suspension was divided randomly into two halves, then one hal f was cultured on microcarriers in FCS system and the other in PPVS system. Five pigs were done separately and by the same method. ① Culture of hepatocytes in FCS system: five flasks were prepared and each one added with 2-3 mL 50 g/L sterile methyl silicon resin ethyl acetate solution. After the flasks being shaken mildly and equably, the remained solution was discarded. The flasks were parched in 60 °C electric oven for use. 20-30 mg Cytodex-3 was deposited into the siliconized flasks and rinsed with 40 mL Ca2+ and Mg2+ free phosphate buffer solution (PBS) for three times, then dipped in PBS over night. After being sterilized at 15 pounds for 30 min, the microcarriers were dipped in DMEM matrix over 10 h for use. Hepatocytes were inoculated into the flasks at the concentration of 5 × 106/mL and DMEM matrix containing 100 mL/L FCS, 100 μg/L glucagon, 100 μg/L transferrin, 200 μg/L hydrocortisone, 100 μg/L insulin and 200 μg/L fortum added up to the total volume, 50 mL. Then the flasks were deposited into an incubator with 100% humidified atmosphere containing 5% CO2 at 37 °C and shaken reelingly once every 15-30 min at the first 4 h, once every 2 h after 4 h and laid quietly after 8 h without further shaking. Ten mL supernatant fluid of the culture matrix was changed after 24 h and once every 2 d from then on. The supernatant fluid was centrifuged at 1000 r/min for discarding the cell fragments. Synthesized albumin and urea were determined. ② Culture of hepatocytes in PPVS system: 100 mL/L refined PPVS was added into the culture system instead of FCS, and the other demands were the same as those of the FCS culture.
Spheroidal aggregate culture of primary porcine hepatocytes Five flasks were prepared and each one added with 2-3 mL 50 g/L sterile methyl silicon resin ethyl acetate solution. After the flasks being shaken mildly and equably, the residual solution was discarded. The flasks were parched in 60 °C electric oven for use. Hepatocytes were inoculated into the flasks at the concentration of 5 × 106/mL and DMEM matrix containing 100 mL/L porcine portal vein serum, 100 μg/L glucagon, 100 μg/L transferrin, 200 μg/L hydrocortisone, 100 μg/L insulin and 200 μg/L fortum added up to a total volume, 50 mL. Then the flasks w ere deposited into the incubator with 100% humidified atmosphere containing 5% CO2 at 37 °C and shaken reelingly once every 15-30 min at the first 4 h, once every 2 h after 4 h and laid quietly after 8 h without further shaking. 10 mL supernatant fluid of the culture matrix was changed afte r 24 h and once every 2 d from then on. The supernatant fluid was centrifuged at 1000 r/min for discarding the cell fragments. Syntheses of albumin and urea were determined.
Morphological observation and counting of hepatocytes The porcine hepatocytes cultured on microcarriers and those cultured as aggregated spheroids were observed under phase- contrast light microscope and photograph ed on an automatic microscopic photographing device successively (Olympus, Japan). In culture on microcarriers 1 mL samples were collected every day to calculate the amount of cells in the culture system by means of trypan blau. On d7, cell sample was taken at a well-distributed state, culture matrix discarded, rinsed with PBS, then fixed for 0.5 h with 2 mL 20 g/L pentanal, rinsed with PBS again, fixed for 0.5 h with 10 g/L osmic acid, and next dehydrated gradientially for 10 min each stage, exchanged with acetic isopental ester for 4 h, dried by CO2 drier (Hitachi HCP2, Japan), and finally splashed with ionic platinum in vacuum. Growth of the cells was observed on S-450 electron scanning microscope (Japan). In spheroidal aggregate culture, 1 mL samples were taken well-distributedly from the matrix for cell calculation on the d7 and d12.
Determination of albumin and urea syntheses When the culture matrix was changed, samples of supernatant fluid were obtained from the culture on microcarriers and spheroidal aggregate culture to determine the contents of albumin and urea through an automatic biochemical analyzer (Beckman, USA).
We successfully took the improved Seglen′s two-step orthotopic perfusion method with collagenase to isolate the porcine hepatocytes. About 2.82 × 1010 hepatocytes were obtained and the survival rate 91.2%. Each gram pig liver offered 7.01 × 107 hepatocytes.
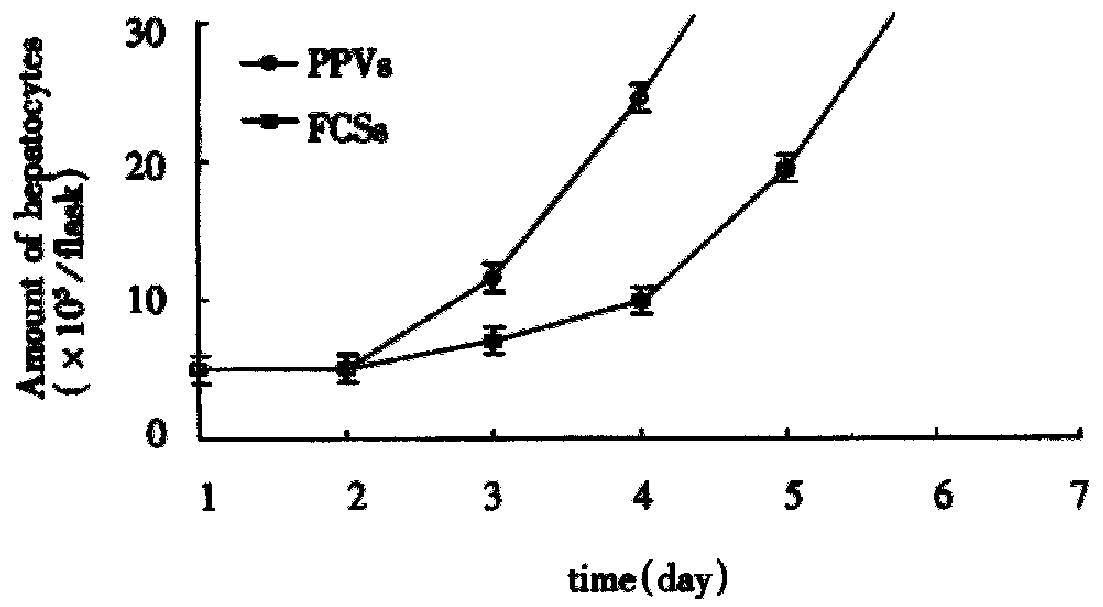
In culture on microcarriers, the amount of hepatocytes on the d7 in the two systems is shown in Table 2. Hepatocytes growth curve (Figure 1) was drawn on the basis of Table 2. On d18 hepatocytes in FCS culture system amounted to (1.1 ± 0.53) × 107 and those in PPVS system (3.1 ± 0.71) × 108. In spheroidal aggregate culture the amount of hepatocytes on d7 was (3.12 ± 0.26) × 107 and that on d10 (1.39 ± 0.51) × 108.
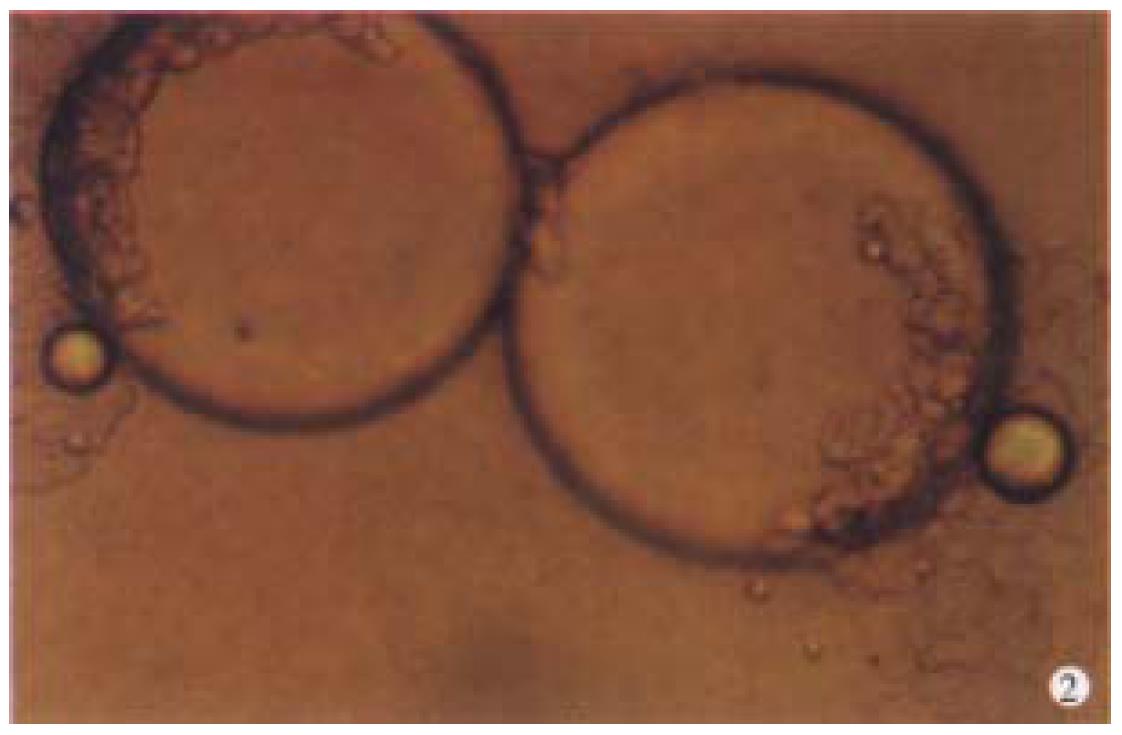
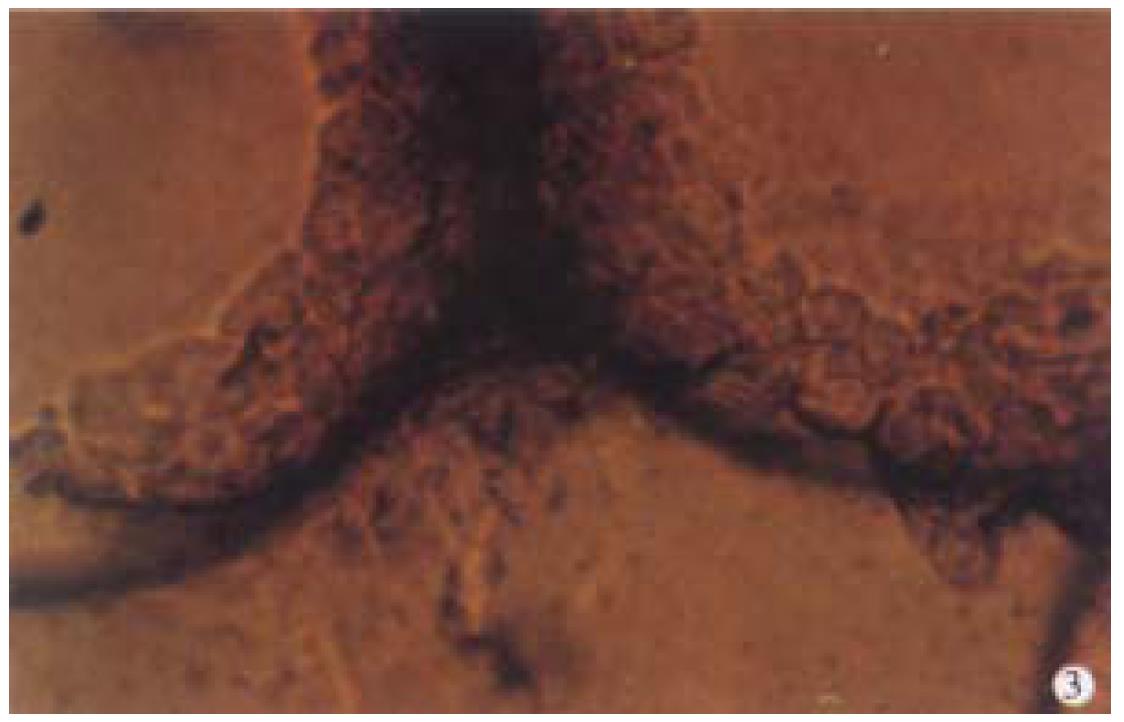
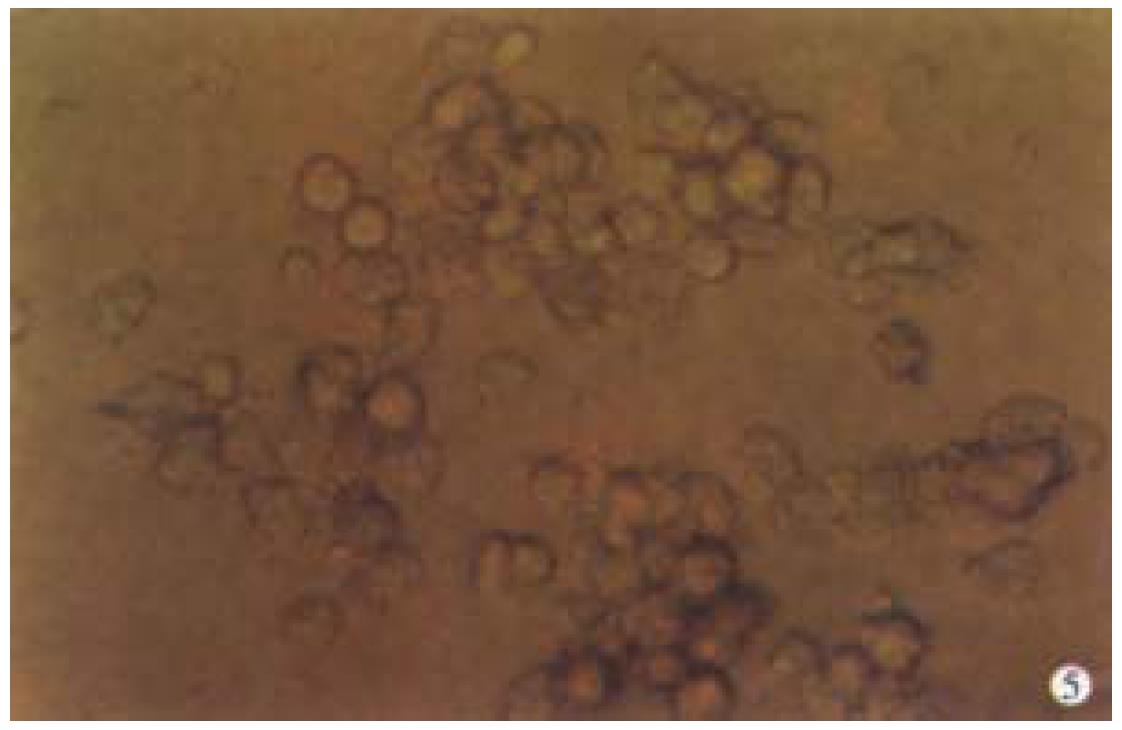
Observation under phase-contrast light microscope Four hours after inoculation microcarriers were observed to be adhered with hepatocytes and the hepatocytes were beginning to expand. The phenomenon of bridge-link could be observed between microcarriers (Figure 2), i.e. the microcarriers were linked with each other through hepatocytes and arranged as slices or a str ing of beads (Figure 3). There was "snowball" effect between some microcarrier s and hepatocytes. Twenty-four hours after culture over 1/3 microcarriers were covered with cells. The hepatocytes adhering to microcarriers was in irregular polygonal shape and thereafter these cells were observed to expand and grow. Forty-eight hours after culture most vivid hepatocytes were attached to micro carriers directly or indirectly. Growth of hepatocytes on microcarriers resemble d that of monolayer growth in flasks. In FCS system hepatocytes began to adhere to microcarriers and expanded 2 h after inoculation. Double-nucleus hepatocytes could be observed and some linked to others in insular configuration. Twenty-four hours later about 60%-70% hepatocytes were attached. Three days after inoculation they began to proliferate and there were anile hepatocytes 6 d later. About 22 d later microcarriers were covered fully with hepatocytes. Five weeks after inoculation hepatocytes tended to become rough and blurry-edged. Granules and vacuoles could be observed in most of them. About 2 d later hepatocytes began to detach from microcarriers and died. Morphology of hepatocytes in PPVS system resembled that in FCS system at the first 24 h except that there were more double-nucleus hepatocytes in PPVS system. Three days after inoculation most microcarriers were covered with neonatal hepatocytes and they linked to others in spheroidal or trabecular conformation. Eighteen days later microcarriers were covered fully with hepatocytes and such condition could be maintained to the d52. Fifty-four days later hepatocytes could be observed to begin falling off from microcarriers and completed on the d56.
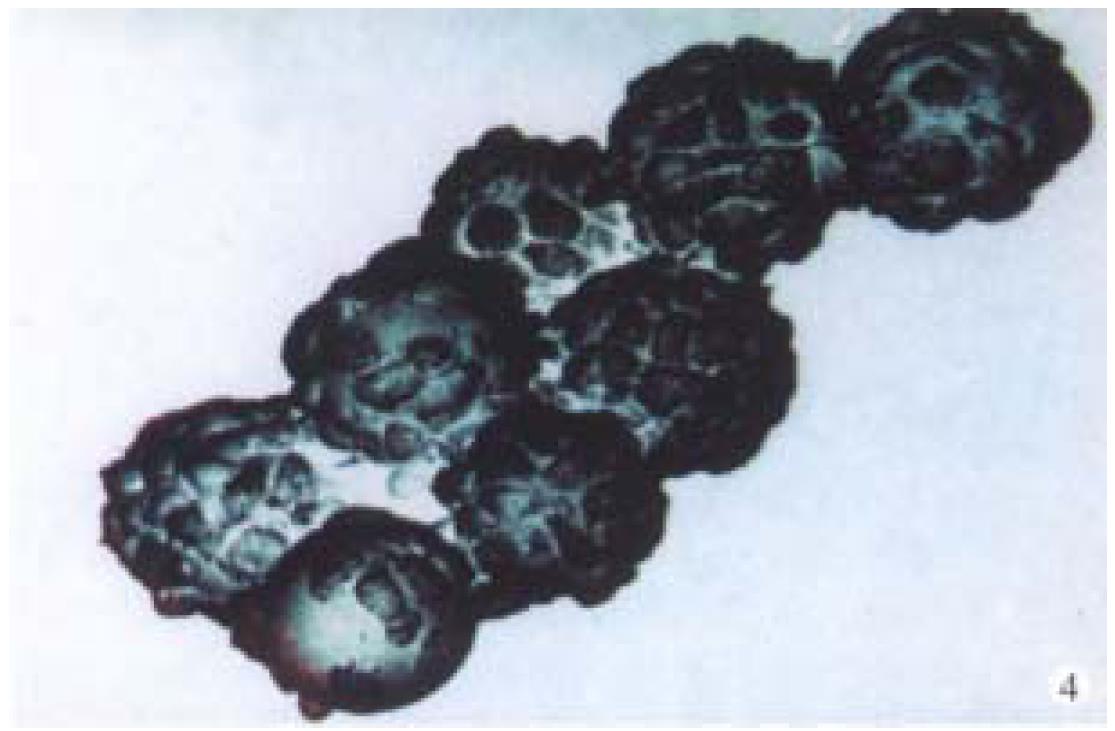
Observation under electron microscope On d18 of culture, the electron microscope showed that the hepatocytes adhering firmly to the microcarriers in semi-spherical form. The microvilli on the surfaces of hepatocytes could be observed clearly. The amount of hepatocytes adhering to the microcarriers varied and they were distributed unevenly. Some microcarriers aggregated with others through cell-bridge as shown in Figure 4.
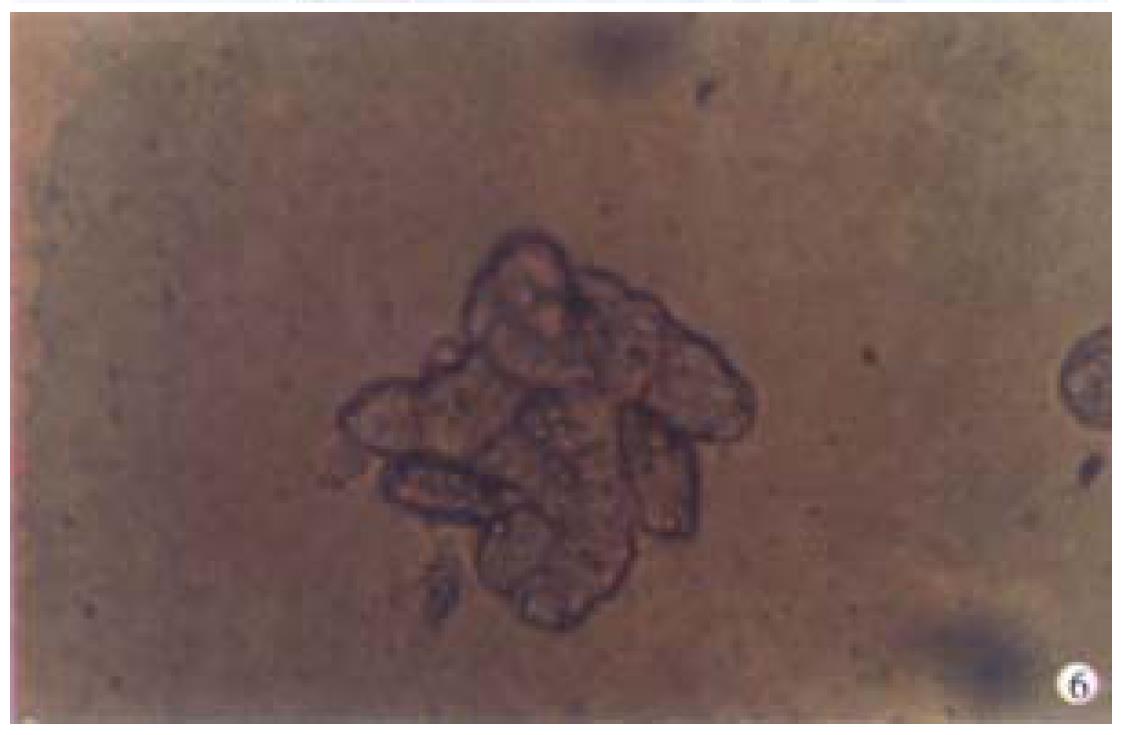
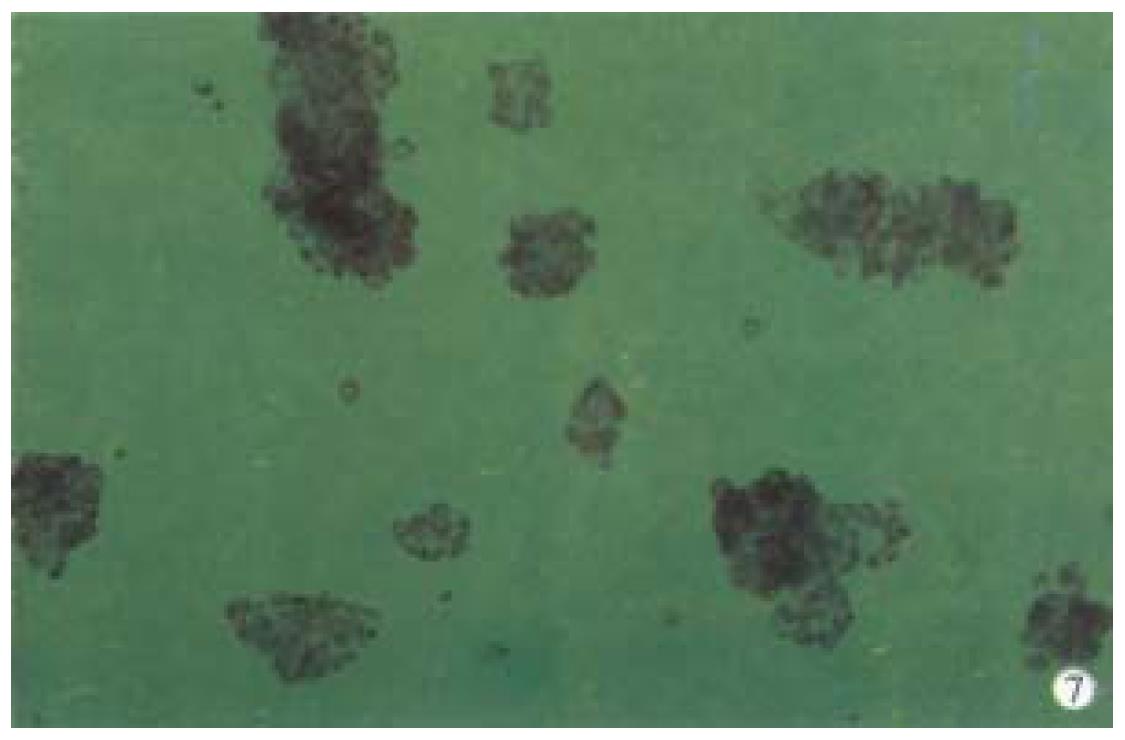
When successively observing the normal primary porcine hepatocytes cultu red in spheroidal-aggregates under the phase-contrast microscope, we found that: about 80%-90% hepatocytes began to aggregate spheroidally 24 h after being inoculated and diameters of the spheroids ranged from 62 μm to 134 μm. Each spheroid contained 10-20 hepatocytes. The hepatocytes at this time linked with each other loosely and were likely to be dispersed. When observed under light microscope, they looked like "cell flowers" (Figure 5). On the d7 they were hyperplas tic and linked with others tightly and looked like "cell spheroids" (Figure 6). On d10 they took the shapes of spheroid and irregular column as "algoid plant" (Figure 7). On the d20, the algoid hepatocytes began to atrophy and vacuolous granules appeared in them, the nuclei began to lyse and the hepatocytes ended in falling off and dying. On the d23, all of the spheroidal aggregating hepatocytes died.
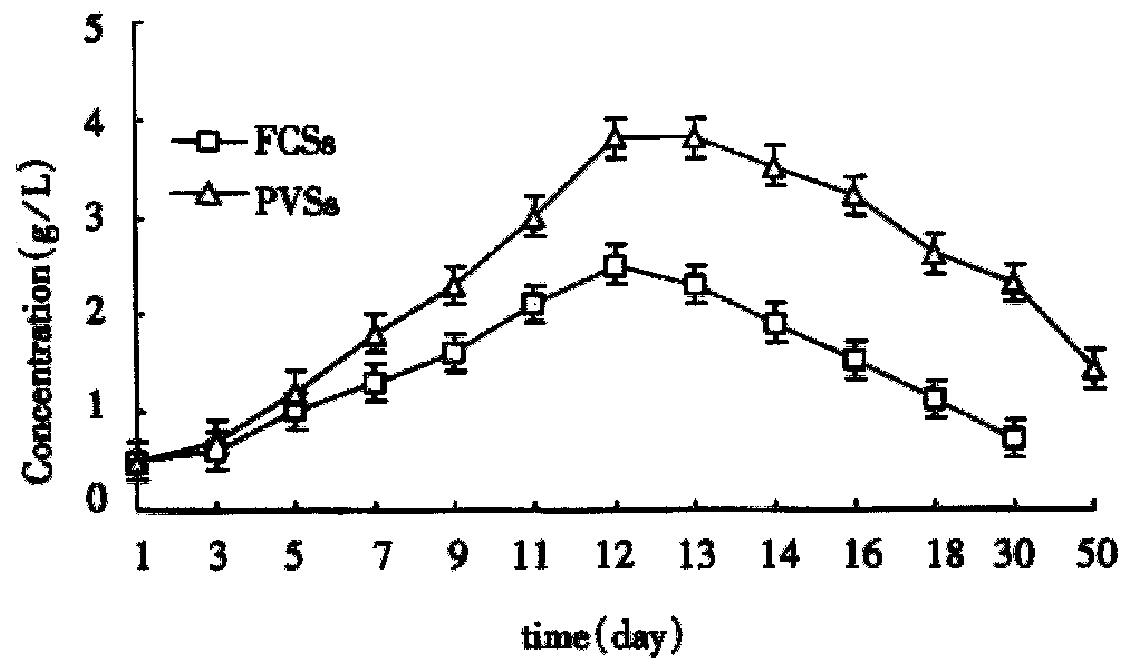
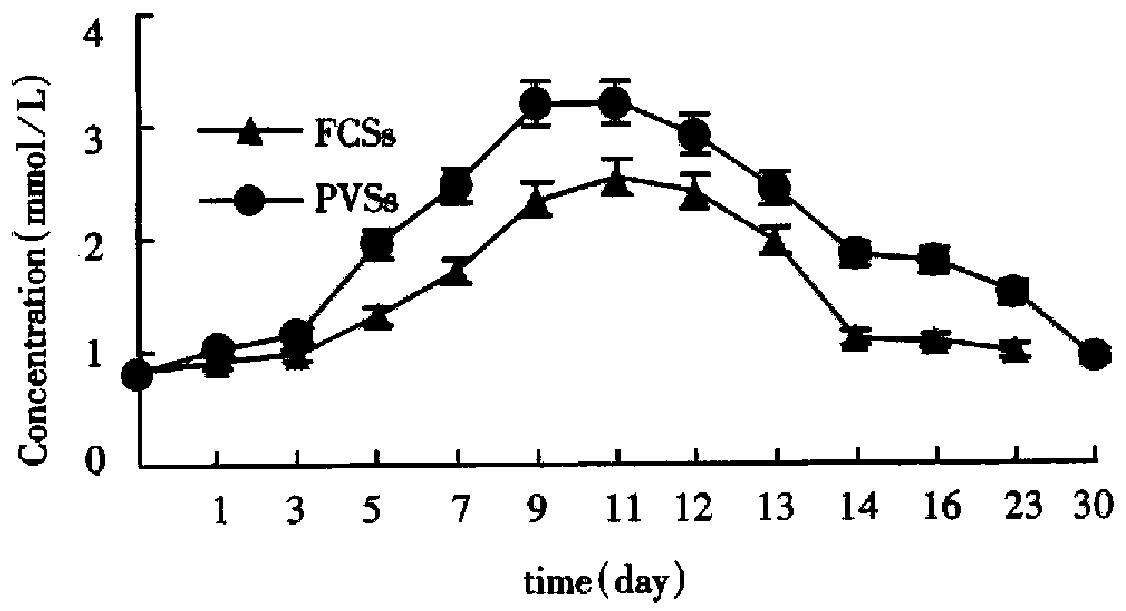
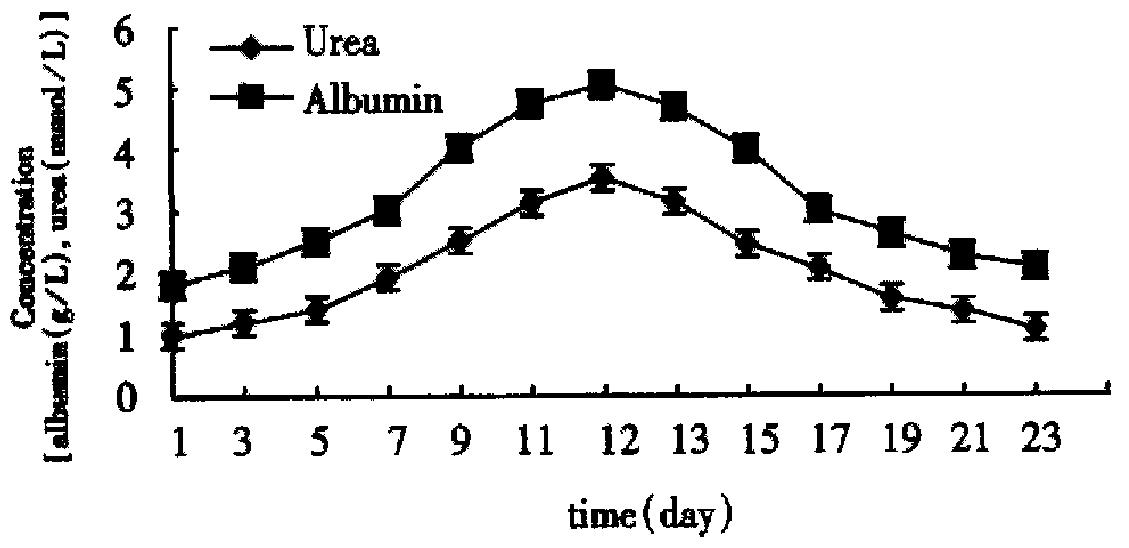
By using the automatic biochemical analyzer (Beckman, U.S.A.) we determined the contents of albumin and urea in the supernatant fluid at different times successively. In the two culture systems on microcarriers the values were kept in high levels from the d 7 to d 14 and got the summit on the d 11 to d 12 (Figure 8 and Figure 9). As Figure 8 and Figure 9 showed, functions of hepatocytes in PPVS system 3-5 d after inoculation were obviously higher than those in FCS system. Hepatocytes in PPVS can maintain their activity for about 50 d. In spheroidal aggregate culture the values were kept in high levels from d 7 to d 14 and got the summit on d 10. From then on, the values degraded gradually and finally the hepatocytes lost their biofunctions on d 23 (Figure 10).
Years of researches at home and abroad showed that primary hepatocytes were the most ideal biomaterial for BAL. But for the morphological and functional changes of primary hepatocytes cultivated in vitro occurred obviously in short time, it was deadly restrained to use them as biomaterial in BAL. The specific functions of hepatocytes lost in in vitro culture accounted for the lack of some in vivo elements. Then it was proposed that syntheses of albumin and urea could be promoted by adding some nutritional factors and hormones into the monolayer culture system. By now some improvements has been achieved in searching for a new effective system to cultivate primary hepatocytes in vitro by the following methods: ①adding hormonal agents such as insulin, glucagon, transferrin an d hydrocortisone into the basal culture medium[1]; ②adding extracellular biological matrix into the culture system[2-4]; ③enriching the culture system with hepatocytes growth stimulating factors such as hepatocytes growth factor and epithelial cell growth factor etc[5,6]; ④co-culturing with other endodermal and mesodermal cells; ⑤three- dimensional cultivation such as microcapsular and macrocapsular culture, spheroid aggregate, microcarrier culture and hollow fiber culture[7,8]; ⑥adding several amino acids and nutritional substances into the culture system; and ⑦adding penetrating-inducer such as pentobarbiturate to the culture system[9]. The above measures all aimed to make a circumstance mimic to the in vivo enviroment for the hepatocytes.
It is well-known that about 70% blood supply of liver comes from portal vein system. The growth of liver and maintainence of its functions depend mainly on the nutritional circumstance provided by the portal vein system, that is, hepatocytes in vivo are norished by the contents of portal vein blood. The later provides various amino acids, energy substances and several growth factors. Our experiments were based on this very point and PPVS was added into the culture matrix. The results showed that hepatocytes in this system could durably survive and maintain their biofunctions. This fluid was the ideal circumstance to cultivate the biomaterial for BAL.
Microcarriers Cytodex-3 were used to provide three-dimensional space for adhering and suspending hepatocytes so as to bring benefit from fully utilizing of the nutritional components in the culture system. Our proceeding studies had prove d that Cytodex-3 was the best microcarrier for primary hepatocytes[3]. Hepatocytes on Cytodex-3 were readily to shape the microstructure similar to that of liver so they could grow and function well.
Landry et al[10] found that when no adhering agent in the spheroidal-aggregate culture system the isolated hepatocytes tended to be changed less in morphology and could survive and maintain their activities for 4-6 wk due to the resemblance of the structure between the aggregating spheroids and normal liver. Clayton et al[11,12] found that the specific transcription gene of hepatocyte in little masses of liver could be kept well than that of isolated hepatocytes and obviously the three-dimension structure of hepatocytes is of great importance in maintaining their specific functions. Relationship between cell skeleton and specific proteins reflected the influences of morphology to functions, that is, cell functions directly related with levels of mRNA and specific proteins, and inversely related with levels of actin and canaliculous protein. Sakai et al[13] found that the inoculation concentration was of great influence to the formation of hepatocyte spheroids in the suspending spheroidal aggregate culture system. When hepatocytes being inoculated at a high concentration it often occurred that pH of culture matrix declined, cell-to-cell and spheroid to spheroid relationships changed and multiple necrotic foci could be ob served in hepatocyte spheroids. When hepatocytes being inoculated at a low concentration they were difficult to aggregate into spheroids. Sakai et al[13] considered 5.0 × 106/mL to be the optimal inoculation concentration in suspending spheroid aggregate culture system of hepatocytes.
On view of the above factors, we concluded that primary porcine hepatocytes cultured on Cytodex-3 in PPVS system or in spheroidal-aggregate were promising to act as the biomaterial of BAL. Still there is much to be desired to promote the density, function and surviving time of hepatocytes for clinical use.
Dr. Yi Gao, graduated from Second Military Medical University as MD in 1992, associate professor of hepatobiliary surgery, mentor of postgraduate.
Edited by You DY
proofread by Sun SM
| 1. | Chen K, Gao Y, Pan YX, Yang JZ. An effective system for culturing primary porcine hepatocytes. Shijie Huaren Xiaohua Zazhi. 1999;7:206-209. |
| 2. | Kimura M, Ogihara M. Proliferation of adult rat hepatocytes in primary culture induced by insulin is potentiated by cAMP-elevating agents. Eur J Pharmacol. 1997;327:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Kono Y, Yang S, Roberts EA. Extended primary culture of human hepatocytes in a collagen gel sandwich system. In Vitro Cell Dev Biol Anim. 1997;33:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Taguchi K, Matsushita M, Takahashi M, Uchino J. Development of a bioartificial liver with sandwiched-cultured hepatocytes between two collagen gel layers. Artif Organs. 1996;20:178-185. [PubMed] |
| 5. | Koike M, Matsushita M, Taguchi K, Uchino J. Function of culturing monolayer hepatocytes by collagen gel coating and coculture with nonparenchymal cells. Artif Organs. 1996;20:186-192. [PubMed] |
| 6. | Schuppan D, Schmid M, Somasundaram R, Ackermann R, Ruehl M, Nakamura T, Riecken EO. Collagens in the liver extracellular matrix bind hepatocyte growth factor. Gastroenterology. 1998;114:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Hu MY, Cipolle M, Sielaff T, Lovdahl MJ, Mann HJ, Remmel RP, Cerra FB. Effects of hepatocyte growth factor on viability and biotransformation functions of hepatocytes in gel entrapped and monolayer culture. Crit Care Med. 1995;23:1237-1242. [PubMed] |
| 8. | Flendrig LM, te Velde AA, Chamuleau RA. Semipermeable hollow fiber membranes in hepatocyte bioreactors: a prerequisite for a successful bioartificial liver? Artif Organs. 1997;21:1177-1181. [PubMed] |
| 9. | Sielaff TD, Nyberg SL, Rollins MD, Hu MY, Amiot B, Lee A, Wu FJ, Hu WS, Cerra FB. Characterization of the three-compartment gel-entrapment porcine hepatocyte bioartificial liver. Cell Biol Toxicol. 1997;13:357-364. [PubMed] |
| 10. | Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914-923. [PubMed] |
| 11. | Clayton DF, Harrelson AL, Darnell JE. Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985;5:2623-2632. [PubMed] |
| 12. | Sawamoto K, Takahashi N. Modulation of hepatocyte function by changing the cell shape in primary culture. In Vitro Cell Dev Biol Anim. 1997;33:569-574. [PubMed] |
| 13. | Sakai Y, Naruse K, Nagashima I, Muto T, Suzuki M. Large-scale preparation and function of porcine hepatocyte spheroids. Int J Artif Organs. 1996;19:294-301. [PubMed] |