Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.353
Revised: February 3, 2000
Accepted: March 1, 2000
Published online: June 15, 2000
AIM: To study the changes of endogenous transforming growth factor β (TGF-β) and basic fibroblast growth factor (bFGF) in lung following intestinal ischemia and reperfusion injury and their effects on lung injury and repair.
METHODS: Sixty Wistar rats were divided into five groups, which underwent sham-operation, ischemia (45 min), and reperfusion (6, 24 and 48 h, respectively) after ischemia (45 min). Immunohistochemical method was used to observe the localization and amounts of both growth factors.
RESULTS: Positive signals of both growth factors could be found in normal lung, mainly in alveolar cells and endothelial cells of vein. After ischemia and reperfusion insult, expressions of both growth factors were increased and their amounts at 6 h were larger than those of normal control or of 24 and 48 h after insult.
CONCLUSION: The endogenous bFGF and TGF-βexpression appears to be up-regulated in the lung following intestinal ischemia and reperfusion, suggesting that both growth factors may be involved in the process of lung injury and repair.
- Citation: Fu XB, Yang YH, Sun TZ, Gu XM, Jiang LX, Sun XQ, Sheng ZY. Effect of intestinal ischemia-reperfusion on expressions of endogenous basic fibroblast growth factor and transforming growth factor β in lung and its relation with lung repair. World J Gastroenterol 2000; 6(3): 353-355
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.353
Our previous investigations have shown that basic fibroblast growth factor (bFGF) and transforming growth factor β (TGF-β) play important roles in organ injury and repair after ischemia and reperfusion insult, and that there was a significant relationship between gene expression of bFGF or TGF-β and lung repair[1,2]. Because many growth factors are involved in wound repair by their mitogenic and non-mitogenic effects, we have further investigated the alteration of endogenous bFGF and TGF-β in the lung tissue following intestinal ischemia-reperfusion injury and explored their effects on lung repair as well.
Sixty male, pathogen-free Wistar rats, weighing 250 g ± 10 g were used in this study. They were divided into 5 groups, which underwent sham-operation, ischemia for 45 min, and reperfusion for 6 h, 24 h and 48 h after ischemia for 45 min, respectively. Anesthesia was induce d by administration of 30 mg/kg of pentobarbital sodium. Following midline laparotomy, intestinal ischemia was achieved by complete occlusion of the superior mesenteric artery (SMA) with a non-crushing microvascular clip. Reperfusion was peformed by removal of the micro vascular clip after 45 min SMA occlusion. All animals were killed by exsangu ination at designated times. The samples from right base of lung were obtained immediately and fixed in 10% formalin for analysis. All procedures but SMA clip were done in animals of sham-operated control group.
Immunohistochemical detection was made using polyclonal anti-bFGF or TGF-β antibody (Santa Cruz Co. and Zymed Co., respectively) by an indirect streptavidin/peroxidase (SP) technique. Experiments were performed following the manufacturer′s recommendation. Paraffin-embedded sections were incubated with polyclonal anti-rat bFGF or TGF-β antibody for 12 h at 4 °Cafter antigen repair. Biotinylated IgG was added as second antibody. Horseradish peroxidase labeled streptomycin-avidin complex was used to detect second antibody. Slides were stained with diaminnobenzidine, and examined under light microscope. The brown or dark brow n stained cytoplasm and/or cell membrane was considered as positive. The phosphate-buffered saline (PBS) solution was used as negative control.
The slides from 5 animals in each group were used for observation and statistical analysis. One visual field in each slide was randomly selected and observed under light microscope with 400-fold magnification. The percentage of positive immunohistoc hemical staining cells was expressed as mean ± SD. Statistical analyses were performed using paired Student′s t test. P < 0.05 was considered significant.
The histological structure of alveolar and mesenchymal cells was normal in healthy lungs, while the lung tissues from ischemia and reperfusion rats were significantly damaged, with pulmonary edema and inflammatory cell infiltration.
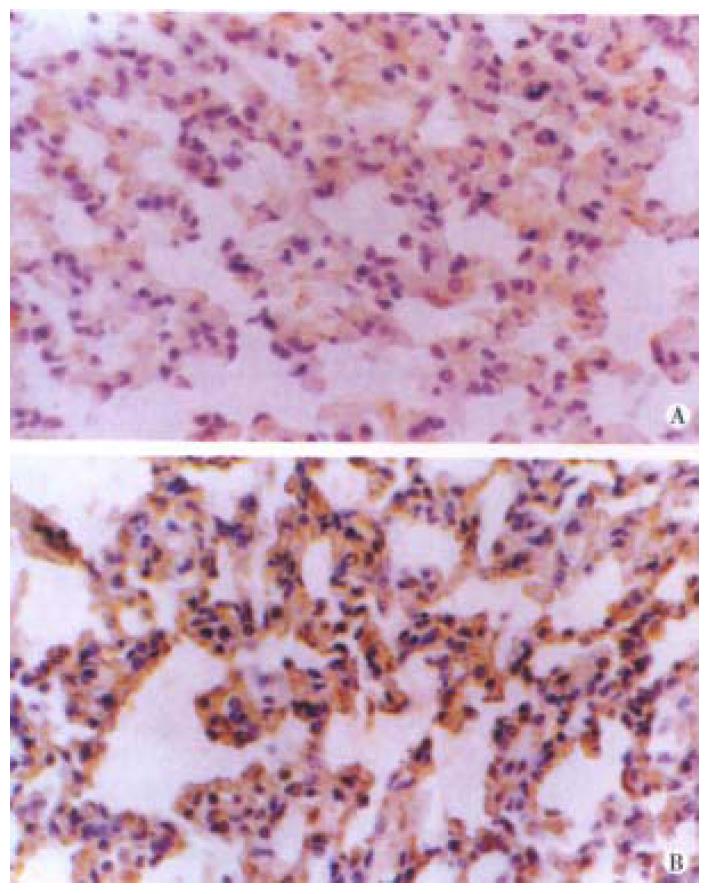
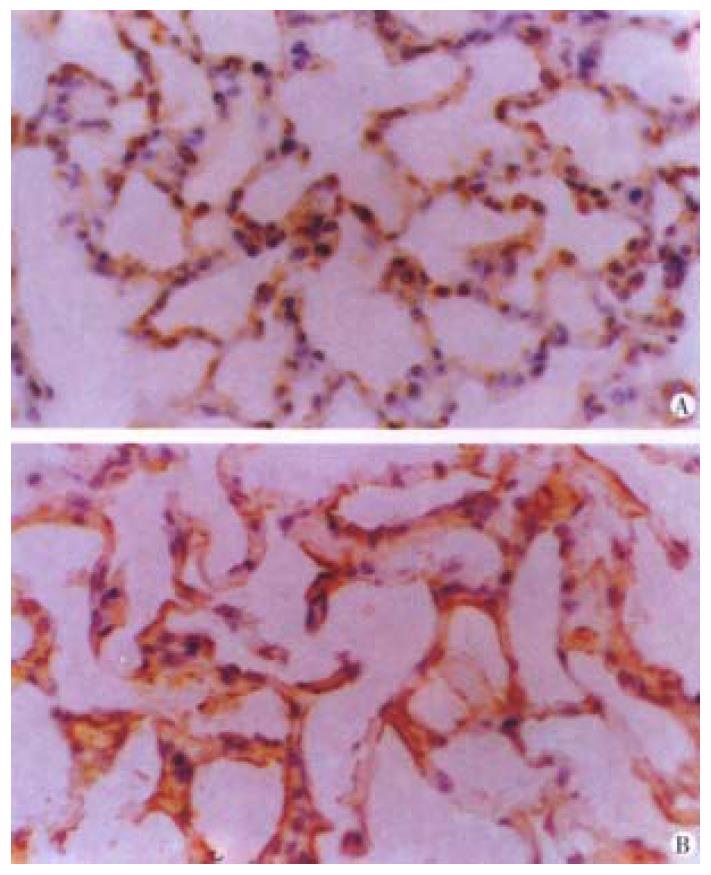
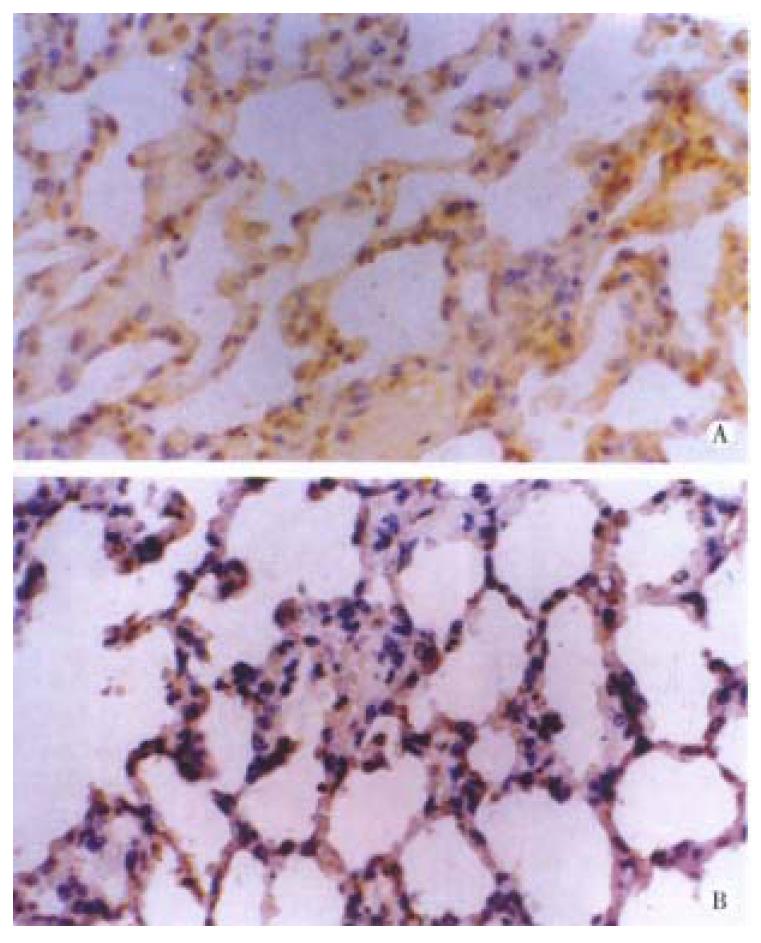
Both bFGF and TGF-β were expressed in alveolar epithelial cells and microvascular endothelial cells of normal lung tissues. The positive signal s were of immunohistochemical staining in brown or dark brown color and localize d in cytoplasm and/or membrane when observed under light microscopy (Figure 1A and B). After ischemia, the expressions of both bFGF and TGF-β were increased, expecially in the area of alveolar epithelial cells and capillary endothelial cells (Figure 2A and B). At 6 h postinjury, the expression of bFGF was the same as that in the early injury, while that of TGF-β was increased significantly. Many positive cells were type I alveolar cells (Figure 3A and B). Up to 24 h and 48 h postinjury, the expression of both growth factors returned to basal levels. By quantitative analysis, the expressions of both bFGF and TGF-β were quite different in the early injury when compared with those of control group (P < 0.01, Table 1).
The lung is one of the very important target organs in multiple organ dysfunction syndrome (MODS) or multiple system organ failure (MOSF) caused by severe injury[3]. It has been found that in addition to the direct trauma, the lung could also be damaged by indirect injury such as shock, gut ischemia, reperfusion insult, etc. Under the condition of an inadequate mucosal blood flow, the gut barrier function can be progressively impaired and invasded by bacteria or endogenous endotoxin. This process is associated with activation of systemic inflammatory mediators including bacteriotoxin, inflammatory mediators, such as tumor necrosis factor (TNF) and interleukin (IL) and immunocytokines. The tissue damage was manifested as increased inflammatory reaction, low content of ATP in tissue, alveolar endothelial cell damage, enhanced permeability of microcirculation, etc[4]. In severe cases, the animal would die of pulmonary failure. But most commonly, these changes are maintained brief because the lung has the ability of self-repair. Recent studies demonstrated that one of the important mechanisms of self-protection and self-repair was the effect of endogenous growth f actor and/or nitric oxide synthetase[2,5]. Therefore, the localization and quantitation of endogenous growth factors play important roles in lung repair.
Both bFGF and TGF-β are important growth factors involved in tissue repair. They are involved in dermal and epidermal wound healing via their chemotactic effects for inflammatory cells and mitogenic effects for tissue cells, such as epidermal cells, fibroblasts and endothelial cells. Normally, TGF-β is stored and released from platelets and macrophages, while bFGF combined with heparin is stored in endothelial cells in an inactive form. Both the growth factors are involved in the process of capillary reconstruction and tissue regeneration by their mitogenic and non-mitogenic effects. At the same time, they can also be relieved from injured tissues[6,7]. Our previous researches have indicated that severe trauma results in histological damage, further decreasing the endogenous growth factors. Thus, it is necessary to supply exogenous growth factors to promote internal organ repair. We have also found that slight ischemia can induce the expression of endogenous factors, and these growth factors participate in the process of wound healing[8,9]. We also investigated the gene expression of both growth factors in the same animal model, and found that the changes of these gene expressions were consistent with the changes of their proteins. On the basis of these studies, we came to a conclusion that there is a positive relationship between growth factors and tissue repair, and induction of endogenous bFGF and TGF-β by ischemia is necessary for tissue repair. By the end of tissue repair, they are restored in tissue again. This result demonstrates that growth factors are involved in organ repair by their increased synthesis or released from damage cells after ischemia-reperfusion insult.
Dr. Xiao-Bing Fu, graduated from the Third Military Medical University as a Master of Medicine in 1988 and University of Madrid, Spain as a Doctor of Medicine, professor and head of research laboratory, majoring in wound healing, multiple organ injury and biology of growth factors, having 160 papers and 3 books published.
Project supported by the National Grant for Outstanding Young Researchers of China, No. 39525024
Edited by You DY and Ma JY
| 1. | Fu XB. Treatment of internal organ damage by growth factors. Xin Xiaohuabingxue Zazhi. 1997;5:663-664. |
| 2. | Yang YH, Fu XB, Sun TZ, Wang YP, Sheng ZY. bFGF and TGF-β gene expression inrat lung after ischemia reperfusion. Jiefangjun Yixue Zazhi. 1998;23:405-409. |
| 3. | Guan J, Jin DD, Jin LJ. [JP2] Apoptosis in rat lung after shock caused by multiple injuries. Zhongguo Weizhongbing Jijiu Yixue. 1998;10:479-483. |
| 4. | Turnage RH, Guice KS, Oldham KT. Endotoxemia and remote organ injury following intestinal reperfusion. J Surg Res. 1994;56:571-578. [PubMed] |
| 5. | Turnage RH, Kadesky KM, Bartula L, Myers SI. Intestinal reperfusion up-regulates inducible nitric oxide synthase activity within the lung. Surgery. 1995;118:288-293. [PubMed] |
| 6. | Muthukrishnan L, Warder E, McNeil PL. Basic fibroblast growth factor is efficiently released from a cytolsolic storage site through plasma membrane disruptions of endothelial cells. J Cell Physiol. 1991;148:1-16. [PubMed] |
| 7. | Sporn MB, Roberts AB. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992;119:1017-1021. [PubMed] |
| 8. | Fu X, Cuevas P, Gimenez-Gallego G, Martinez-Murillo R, Tian H, Sheng Z. Ischemia and reperfusion reduce the endogenous basic fibroblast growth factor in rat skeletal muscles: an immunohistochemical study. Wound Repair Regen. 1996;4:381-385. [PubMed] |
| 9. | Fu X, Yang Y, Li X, Sun T, Wang Y, Sheng Z. Ischemia and reperfusion impair the gene expression of endogenous basic fibroblast growth factor (bFGF) in rat skeletal muscles. J Surg Res. 1998;80:88-93. [PubMed] |