Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.330
Revised: April 3, 2000
Accepted: April 25, 2000
Published online: June 15, 2000
AIM: To investigate the interaction of Zot with microtubule.
METHODS: Zot affinity column was applied to purify Zot-binding protein(s) from crude intestinal cell lysates. After incubation at room temperature, the column was washed and the proteins bound to the Zot affinity column we re eluted by step gradient with NaCl (0.3-0.5 mol·L-1). The fractions were subjected to 6.0%-15.0% (w/v) gradient SDS-PAGE and then transferred to PVDF membrane for N-terminal sequencing. Purified Zot and tau protein were blotted by using anti-Zot or anti-tau antibodies. Finally, purified Zot was tested in an in vitro tubulin binding assay.
RESULTS: Fractions from Zot affinity column yielded two protein bands with a Mr of 60 kU and 45 kU respectively. The N-terminal sequence of the 60 kU band resulted identical to β-tubulin. Zot also cross-reacts with anti-tau antibodies. In the in vitro tubulin binding assay, Zot co-precipitate with Mt, further suggesting that Zot possesses tubulin-b inding properties.
CONCLUSION: Taken together, these results suggest that Zot regulates the permeability of intestinal tight junctions by binding to intracellular Mt, with the subsequent activation of the intracellular signaling leading to the permeabilization of intercellular tight junctions.
- Citation: Wang WL, Lu RL, DiPierro M, Fasano A. Zonula occludin toxin, a microtubule binding protein. World J Gastroenterol 2000; 6(3): 330-334
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/330.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.330
Vibrio cholerae, the human intestinal pathogen responsible for the diarrhoeal disease cholera, elaborates a large number of extracellular proteins, including several virulence factors. The severe dehydrating diarrhoea characteristic of cholera is induced by cholera toxin (CT). A number of epidemiological studies[1-4] have shown a concurrent occurrence of the CT genes (ctx-A and ctx- B) and the genes for two other virulence factors elaborated by V. cholerae, zonula occludens toxin (Zot)[5] and accessory cholera toxin (Ace)[6]. Zot increases the intestinal permeability by rearranging the intestinal cell cytoskeleton strategically located to modulate intercellular tight junctions[7]. However, the first step of Zot signaling after the protein internalization remains to be established.
Microtubules (MT) are intracellular structures functionally and anatomically rel ated to the cell cytoskeleton. MTs are composed of α-tubulin and β-tubulin. Factors known to regulate microtubule dynamic include microtubule-associated proteins (MAPs). Neuronal MAPs, the most abundant of which are MAP2 and tau, stimu late MT assembly[8,9]. The best characterized function of MT network polymers is the bi-directional movement of membrane vesicles driven by the MT-bas ed motor proteins, kinesin and cytoplasmic dynein. Different cargoes can be transported via MT-dependent vesicles, including various types of endocytic and exocytic vesicles[10,11]. Connection of actin filament network has been found[12]. In eukaryotic organisms, various cell functions, including cell shape and mobility require coordinatial interaction between actin and MT cytoskeleton[13].
In our study, we found that when cell lysates from mammalian tissues passed through a Zot affinity column, two proteins bound to Zot, the N-terminal sequence of one of these two proteins revealed that it corresponded to tubulin; Zot cross-reacted with antibody against Tau, another well described MAP isolated from mammalian brain; by using a microtubule binding assay, Zot was found co-precipitated with MT. Taken together, these results suggest that Zot is a new member of MAPs family. This Zot property may be involved in the Zot signaling leading to the regulation of intercellular TJ.
Plasmid pSU111, containing the clone Zot gene in a pQE-30 vector with a 6-histidine tag at its N-terminal, was grown in LB medium with 20 g/L glucose, 25 μg/L kanamycin and 200 μg/L ampicillin at 37 °C with vigorous mixing until the A600 reached 0.7-0.9. Cultures were then induced with 2 mmol·L-1 isopropylthio-β-D-galactoside (IPTG, Fisher), followed by an additional 2 h culture period at 37 °C with vigorous shaking. The cells were harvested by centrifugation at 4000 ×g for 20 min and resuspended in buffer A (6 mol·L-1 Guanidine-HCl, 0.1 mol·L-1 Na-phosphate, 0.01 mol·L-1 Tris-HCl, pH8.0; 5 mL/g wet cell weight). After stirring for 1 h at room temperature, the mixture was centrifuged at 10000 ×g for 30 min at 4 °C. A 50% slurry of Superflow (QIAGE N, 1 mL/g wet cell) was added to the supernatant and stirred for 1 h at room temperature. The mixture was loaded onto a 5 cm ± 1.5 cm column and washed sequentially with buffer A, buffer B (8 mol·L-1 urea, 0.1 mol·L-1 Na-phosphate, 0.01 mol·L-1 Tris-HCl, pH8.0) and buffer C (8 mol·L-1 urea, 0.1 mol·L-1 Na-phosphate, 0.01 mol·L-1 Tris-HCl, pH6.3). Each wash step was con tinued until the A280 of the flow-through was less than 0.01. His-Zot was eluted by addition of 250 mmol·L-1 imidazole (1,3-diaza-2,4-cyclopentadiene) to buffer C. After dialysis against urea, the eluate was diluted 200-500 times in PBS, stirred with 50% slurry Superflow (1 mL/g wet cell weight) for 2 h at room temperature, loaded onto another 5 cm ± 1.5 cm column, washed with phosphate-buffered saline (PBS) and eluted with 250 mmol·L-1 imidazole in PBS. Purity of the His-Zot protein was established by sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) analysis and Western blot using polyclonal anti-Zot antibodies.
SDS-PAGE It was carried out on a 50-150 g·L-1 gradient gel, stained with Coomassie brilliant blue dye, destained by 75 mL·L-1 acetic acid with 100 mL·L-1 methanol and dried with Gel Drying Film (Promega).
Western blot analysis Following SDS-PAGE, proteins were transferred onto polyvinylidene difluoride (PVDF) membrane (MILLIPORE). Non-specific binding sites were blocked by PBS with 50 mL·L-1 milk plus 1 g·L-1 Tween-20. Primary and secondary antibodies were rabbit polyclonal anti-Zot antibody and anti-rabbit IgG (peroxidase conjugate, Sigma), respectively. Films were exposed with ECL detection reagent (Amersham) for 1 min, and developed by Konica SRX-101 developer.
One mg of his-Zot in 4 mL coupling buffer (0.1 mol·L-1 sodium phosphate, 0.15 mol·L-1 NaCl, pH7.2) and 40 μL of 5 mol·L-1 sodium cyanoborohydride, were added to an equilibrated AminoLink plus column (Pierce) and gently mixed overnight at 4 °C. After washing with coupling buffer, 4 mL of 1 mol·L-1 Tris-HCl (pH7.4) and 40 μL of 5 mol·L-1 sodium cyanoborohydride were added to the column followed by gently mixing for 30 min at room temperature to block the remaining active sites. The column was washed with 1 mol·L-1 NaCl and stored in PBS containing 0.5 g·L-1 sodium azide.
Adult human brain tissues was obtained from the Brain and Tissue Banks for Developmental Disorders at the University of Maryland and used under the approval of the University's Institutional Review Board. Adult human heart and intestinal tissues were utilized for comparative analysis. Tissues were washed with buffer D (20 mmol·L-1 Tris-HCl, 20 mmol·L-1 EDTA, 250 mmol·L-1 sucrose, pH7.5), homogenized in buffer E (buffer D containing 5 mg·L-1 leupeptin, 2 mg·L-1 aprotine, 1 mg·L-1 pepstain, 10 mg·L-1 phenylme thyisulfonyl fluoride (PMSF), and centrifuged at 5000 ×g, 4 °C for 10 min. Supernatants were centrifuged at 12000 ×g, 4 °C for 45 min. Precipitates were discarded and supernatants were centrifuged at 30000 ×g, 4 °C for an additional 90 min. Precipitates were dissolved in buffer E with 5 g·L-1-3[(3-cholamidopropyl) dimetylammonio]-1-propanesulfonate (CHAPS), sitting on ice for 60 min with gentle mixing every five minutes.
Membrane preparations obtained from human brain was loaded on an AminoLink plus-Zot affinity column, washed, and equilibrated with PBS at room temperature containing 1 g·L-1 Triton X-100. The columns were incubated for 90 m in at room temperature, washed with 8 volumes of PBS containing 1 g·L-1 Triton X-100, and eluted with PBS containing 1 g·L-1 Triton X-100 with 0.1 mol·L-1 NaCl, 0.3 mol·L-1 NaCl, 0.5 mol·L-1 NaCl, 0.8 mol·L-1 NaCl and 1.0 mol·L-1 NaCl, respectively. Fractions were collected and subjected to SDS-PAGE. N-terminal amino acid sequence analysis.
The fractions of human tissue lysates containing Zot binding proteins were resolved by 50-150 g·L-1 gradient SDS-PAGE and transferred onto PVDF membranes using CAPS buffer [10 mmol·L-1-3-(cycloh exylamino)-1 propanesulfonic acid and 100 mL·L-1 methanol]. The protein bands were excised and subjected to N-terminal sequencing using a Perkin-Elmer Applied Biosystems Apparatus Model 494.
Spin-Down assay kit (Cytoskeleton) was used in the experiments according to the manufacture's recommendations. MT(20 nmol·L-1 final concentration) were obtained by mixing an aliquate of tubulin (20 μL, 5 g/L) and 2 μL, 200 mmol·L-1 taxol in G-PEM buffer (80 mmol·L-1 Pipes pH6.8, 1 mmol·L-1 MgC l2, 1 mmol·L-1 EGTA and 1 mmol·L-1 GTP). One ug of Zot was incubated with 20 nmol·L-1 microtubule at room temperature for 20 min, while MT-associated protein MAP2 and bovine serum albumin (BSA) were used as positive and negative control, respectively. Proteins attached to MT and unbound proteins were separat ed by centrifugation. Each reaction product was carefully placed on the top of a cushion (G-PEM buffer plus 400 g·L-1 glycerol) in Ultraclear TM ultracentrifuge tube. Following centrifugation, supernants and pellets were carefully removed and supernants were mixed with 1/20th volum of 500 g·L-1 TCA solution for precipatation protein using centrifuge. Both supernants and pellets of the preparations containing the tested proteins were analized by either SDS-PAGE (MAP2 and BSA) or western blotting (Zot).
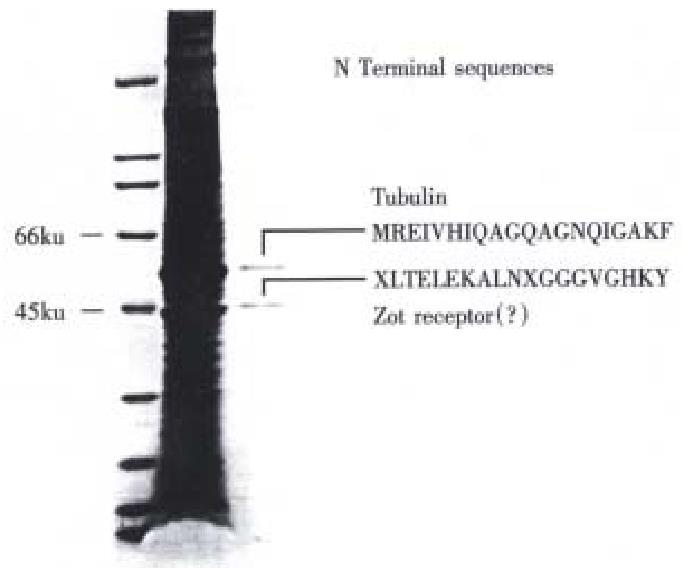
His-Zot was successfully immobilized to AminoLink Plus gel with immobilization yields of 89%-95%, as established by the protein assay (Bio-Rad detergent-compatible protein assay). Plasma membrane preparations from human brain loaded on the Zot affinity column contained two major Zot-binding proteins with apparent molecular masses of approximately 45 kU and 55 kU, respectively (Figure 1, lane 2).
The N-terminal sequences of the two Zot/zonulin binding proteins are shown in Table 1. The two proteins were also compared to other protein sequences by Blast search analysis. The N-terminal sequence of the 55 kU protein was 100% identical to the N- terminal sequence of tubulin (Table 1) whereas the ~45 kU protein band resulted 72% identical to the N-terminus of calprotectin, a calcium b inding protein associated to chronic inflammatory processes[14] and the cystic fibrosis antigen (CFA)[15]. This second protein resulted to be th e Zot/zonulin brain receptor[16].
| Sample | N-terminus | Identity (%) |
| Zot binding protein~55 kU | MREIVHIQAGQAGNQIGAKF | |
| β-tubulin | MREIVHIQAGQAGNQIGAKF | 100 |
| Zot binding protein~45 kU | LTELEKALNXGGGVGHKY | |
| Calprotectin (MRP-8) | LTELEKALNSIIDVYHKY | 77 |
| Cystic fibrosis antigen | LTELEKALNSIIDVYHKY | 77 |
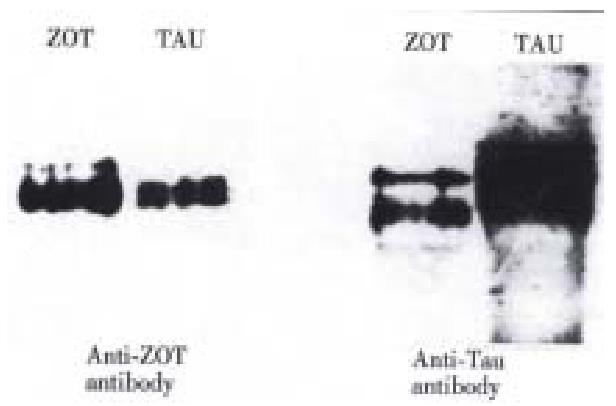
To investigate wheather Zot and tau (a well characterized MAP) are immunologically related, cross immunoscreening experiments were performed. As shown in Figure 2, both proteins were recognized by either anti-Zot antibodies (left pan el) or anti-tau antibodies (right panel). These results suggest that Zot and tau are immunologically related.
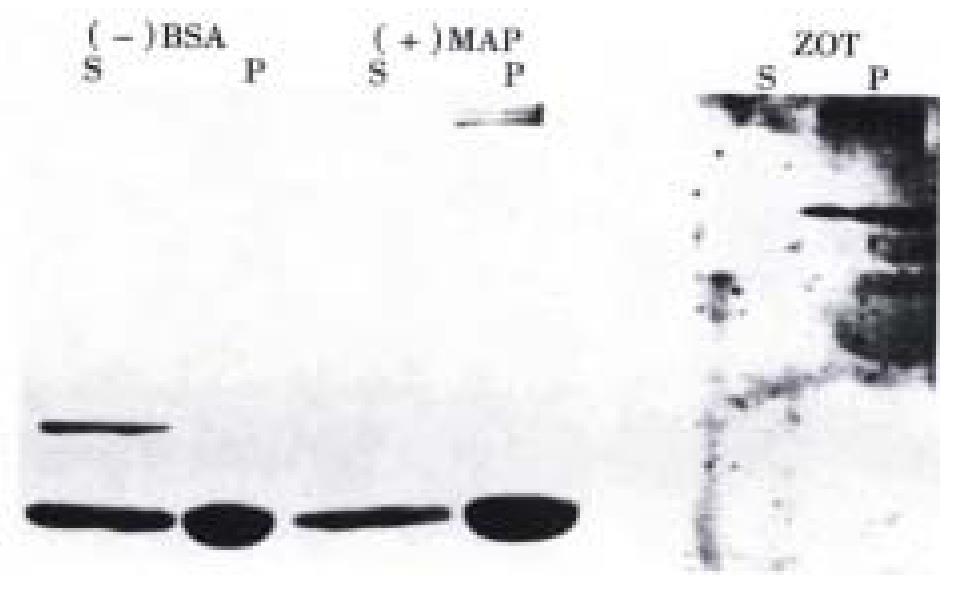
To confirm that Zot possesses MT binding properties, an in vitro binding ass ay was performed. As shown in Figure 3, we observed that Zot co-precipitated with MT as shown by Western immunoblotting analysis, while no Zot was found unboun d. BSA lacking MT-binding properties was present only in the supernant. MAP2, a well defined MT associated protein, co-precipitated with MT and was not present in the supernant. These results confirmed the Zot ability to bind to MT.
Tj is the hallmark of absorptive and secretory epithelia. As a barrier between apical and basolateral compartments, the tj selectively controls the paracellular passage of water, solutes and immune cells between epithelial and endothelial cells. Variations in transepithelial conductance can usually be attributed to changes in the permeability of the paracellular pathway, since the resistances o f eukaryotic cells plasma membrane are relatively high[17]. Tj represent the major barrier in this paracellular pathway and the electrical resistance of epithelial and endothelial tissues seems to depend on the number of transmembrane protein strands and their complexity as observed by freeze-fracture electron microscopy[18]. It has become abundantly clear that, in the presence of Ca2+, assembly of the tj is the result of cellular interactions that trigger a complex cascade of biochemical events that ultimately lead to the f ormation and modulation of an organized network of tj elements, the composition of which has been only partially characterized[19].
Identification and characterization of Zot, a toxin produced by Vibrio cholerae, has provided new information on the regulation of intercellular tj[5,7,20-22]. After binding to its surface receptor, Zot is internalized[23], and subsequently triggers a series of intracellular events including phosp holipase C and PKCα-dependent actin polymerization which leads to the opening of tj[7]. However, the complete cascade of the intracellular events activated by Zot, particularly concerning the early steps, remains undefined. There is now a large body of evidience that protein phosphorylation plays a major role in tj development[24] and cytoskeleton rearrangement[25]. In eukaryotic cells, junctional complex proteins, actin filaments, microtubules, and i ntermediate filaments interact to form the cytoskeleton network involved in dete rmination of cell architecture, intracellular transport, modulation of surface r eceptors, paracellular permeability, mitosis, cell motility, and differentiation [26]. We have previously demonstrated that there are two Zot binding proteins in the cell lysates of Zot-sensitive tissues[16]. One has been characterized as the Zot/zonulin receptor. With this paper we showed that tubulin is the second Zot-binding protein. Based on these results, it is possible t o hypothesize that Zot affects the actin filament network by binding to MT. The association of Zot to MT could be responsible of the effects of Zot on cell uptake and intracellular trafficking of molecules[10,11]as well as the changes of tj structure and permeability. Alterations of intestinal tj occur in a variety of clinical conditions affecting the gastrointestinal system, including food allergies, malabsorption sysndromes, and inflammatory bowel diseases. The knowledge that can eventually be acquired by studying the regulation of tj may have a tremendous impact on our understanding of the pathogenesis of these disease. It would not be surprising if the modification of tj structure and function by these pathological conditions would be an extention of normal physiologic regulation of tj.
However, several questions remain unanswered: what is the role of Zot-MT inter action on rearrangement of actin filament? Does this interaction affect the peameabilty of tj Are MT-dependent cell functions, such as redistribution of organelles and the polarized distribution of membrane proteins, influenced by the MT-Zot binding? Experiments aimed at addressing these questions are presently in progress in our laboratory.
Wen-Le Wang obtained his medical degree from Norman Bethune University of Medical Sciences in 1987 and M.S. in Biochemistry at West China University of Medical Sciences in 1993. He had worked in medical university and institute for three years in China. He is currently a PhD candidate in the Program of Biochemistry and Molecular Biology at University of Maryland, Baltimore. He has 8 papers published.
Edited by Pan BR
proofread by Ma JY
| 1. | Colombo MM, Mastrandrea S, Santona A, de Andrade AP, Uzzau S, Rubino S, Cappuccinelli P. Distribution of the ace, zot, and ctxA Foxin genes in clinical and environmental Vibrio cholerae. J Infect Dis. 1994;170:750-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Johnson JA, Morris JG, Kaper JB. Gene encoding zonula occludens toxin (zot) does not occur independently from cholera enterotoxin genes (ctx) in Vibrio cholerae. J Clin Microbiol. 1993;31:732-733. [PubMed] |
| 3. | Karasawa T, Mihara T, Kurazono H, Nair GB, Garg S, Ramamurthy T, Takeda Y. Distribution of the zot (zonula occludens toxin) gene among strains of Vibrio cholerae 01 and non-01. FEMS Microbiol Lett. 1993;106:143-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Kurazono H, Pal A, Bag PK, Nair GB, Karasawa T, Mihara T, Takeda Y. Distribution of genes encoding cholera toxin, zonula occludens toxin, accessory cholera toxin, and El Tor hemolysin in Vibrio cholerae of diverse origins. Microb Pathog. 1995;18:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD, Ketley JM, Kaper JB. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242-5246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 359] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Trucksis M, Galen JE, Michalski J, Fasano A, Kaper JB. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc Natl Acad Sci USA. 1993;90:5267-5271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 150] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper JB, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum SE. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest. 1995;96:710-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 253] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Murphy DB, Johnson KA, Borisy GG. Role of tubulin-associated proteins in microtubule nucleation and elongation. J Mol Biol. 1977;117:33-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 178] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Sloboda RD, Dentler WL, Rosenbaum JL. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976;15:4497-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 323] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Goodson HV, Valetti C, Kreis TE. Motors and membrane traffic. Curr Opin Cell Biol. 1997;9:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 145] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Cole NB, Lippincott-Schwartz J. Organization of organelles and membrane traffic by microtubules. Curr Opin Cell Biol. 1995;7:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 249] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Fuchs E, Yang Y. Crossroads on cytoskeletal highways. Cell. 1999;98:547-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93:255-266. [PubMed] |
| 14. | Odink K, Cerletti N, Brüggen J, Clerc RG, Tarcsay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 536] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Dorin JR, Novak M, Hill RE, Brock DJ, Secher DS, van Heyningen V. A clue to the basic defect in cystic fibrosis from cloning the CF antigen gene. Nature. 1987;326:614-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Lu R, Wang W, Uzzau S, Vigorito R, Zielke HR, Fasano A. Affinity purification and partial characterization of the zonulin/zonula occludens toxin (Zot) receptor from human brain. J Neurochem. 2000;74:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Diamond JM. Twenty-first Bowditch lecture. The epithelial junction: bridge, gate, and fence. Physiologist. 1977;20:10-18. [PubMed] |
| 18. | Madara JL. Loosening tight junctions. Lessons from the intestine. J Clin Invest. 1989;83:1089-1094. [PubMed] |
| 19. | Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1-F9. [PubMed] |
| 20. | Baudry B, Fasano A, Ketley J, Kaper JB. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect Immun. 1992;60:428-434. [PubMed] |
| 21. | Fasano A, Uzzau S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest. 1997;99:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Fasano A, Uzzau S, Fiore C, Margaretten K. The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology. 1997;112:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Fasano A. Modulation of intestinal permeability: an innovative method of oral drug delivery for the treatment of inherited and acquired human diseases. Mol Genet Metab. 1998;64:12-18. [PubMed] |
| 24. | Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Tang DG, Timar J, Grossi IM, Renaud C, Kimler VA, Diglio CA, Taylor JD, Honn KV. The lipoxygenase metabolite, 12(S)-HETE, induces a protein kinase C-dependent cytoskeletal rearrangement and retraction of microvascular endothelial cells. Exp Cell Res. 1993;207:361-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | MacRae TH. Towards an understanding of microtubule function and cell organization: an overview. Biochem Cell Biol. 1992;70:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |