Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.295
Revised: June 6, 1999
Accepted: June 18, 1999
Published online: April 15, 2000
- Citation: Huang PL, Zhu SN, Lu SL, Dai ZS, Jin YL. Inhibitor of fatty acid synthase induced apoptosis in human colonic cancer cells. World J Gastroenterol 2000; 6(2): 295-297
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/295.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.295
The treatment of human epithelial malignancies is limited by drug resistance and toxic and side effects, which results in the failure in the treatment of majority of advanced cancer victims. To seek for a new, and specific antineoplastic therapy will provide hope for tumor treatment. Although disordered intermediary metabolism in cancer cells has been known for many years, much of the work focused on abnormal glucose catabolism. At the same time, little attention has been paid to fatty acid synthasis in tumor tissues, dispite of the significance of fatty acid synthase (FAS) in some clinical human ovarian[1], breast[2], colorectal[3], and prostatic cancers[4,5]. Tumor cells which express high levels of fatty acid synthesizing enzymes use endogeneously synthesized fatty acids form embrance biosynthesis and appear to export large amounts of lipid. In contrast, normal cells preferentially utilize diary lipid.
The mechanism by which inhibition of fatty acid synthesis produces its antitumor effects remains unexplained. In this study, we studied the effect of fatty acid synthase inhibitor-cerulenin, on the proliferation and apoptosis of colonic cancer cells.
Cell lines and culture condition LoVo cells were maintained in RPMI 1640 with 10% fetal bovine serum. Cultures were incubated at 37 °C in a humidified 5% CO2 atmosphere. A 5 g/L stock solution of cerulenin (Sigma Chemical Co.) in DMSO was diluted to a final concentration of 5 mg/L or 10 mg/L. Human fibroblasts (collected from clinical operation) were cultured in RPMI 1640 for six generations, and then were maintained in the same growth medium as LoVo cells.
Morphological evaluation Exponentially growing LoVo cells were incubated with or without cerulenin in RPMI1640 for 24 h and collected after trypsine was digested. The cells were spun onto glass slides, fixed in methanol, and stained by Wright-Giemsa’s method. The slides were observed under Olympus light miscoscope.
Clonogenic assay Using limiting dilution method, LoVo cells were inoculated at 50 colony-form cells/well into 96-well plates and incubated for 10 d, with triplicate cultures plated at each drug concentration (1.0 × 10-5 mol/L-1.0-9 mol/L). Colonies of greater than 50 cells were counted for all clonogenic assays. The rate of colony forming = (average colony number/planted single cell number) × 100%. The rate of colony forming inhibition = (control colony forming rate-experimental colony forming rate)/control colony forming rate × 100%.
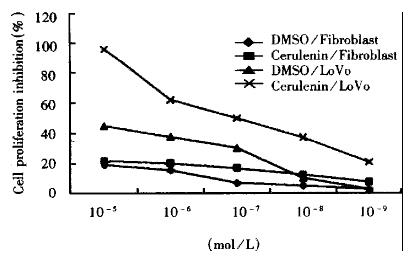
Cell proliferation inhibition experiment (MTT) LoVo cells were inoculated at 8 × 104 cell/mL into 96-well plates and incubated 24 h. Cerulenin of 1.13 × 10-5 mol/L-1.13 × 10-9 mol/L in concentration was given to the experimental group. Each well was added with 20 μL (5 g/L) MTT and incubated for 4 h. The plates were read on Dynatech microplate reader MR600 at 570 nm.
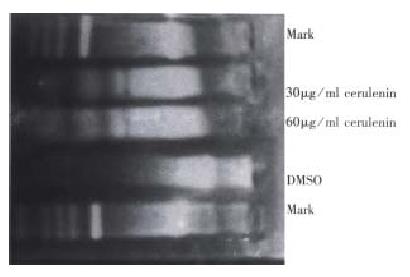
DNA fragmentation assay LoVo cells were incubated with or without cerulenin in RPMI1640 for 12 h and collected after trypsin was digested. At harvest, medium and trysinized cells were combined, and DNA was extracted from pelleted cells and subjected to 1.5% agarose gel. DNA was electrophoresed for 3 h at 5 V/cm. The results were observed and photographed by UV light.
The effects of fatty acid synthase inhibitor on cell growth were examined by MTT method. The inhibitory effects on proliferation are shown in Figure 1. Cerulenin can inhibit significantly the proliferation of LoVo cells and showed dose-response relationship. Therefore, FAS inhibitor-cerulenin might suppress the proliferation of human colonic cancer cells.
To assess the cellular consequences of fatty acid synthesis inhibition, the clonogenicity of LoVo cell was determined after a 10-day exposure to cerulenin (Table 1). LoVo cell line exhibited dose-dependent reduction in clonogenic potential.
The cytotoxic effects of cerulenin must occur within the context of cellular response. A wide variety of cellular injuries in cell death by initiating a cellular program for autodestruction, or apoptosis. Fragmentation of genomic DNA to high molecular weight (180 kb-200 kb) fragments is a characteristic early event in apoptosis and may represent the committed step of the process. Agarose gel electro phoresis was used to evaluate whether high molecular DNA fragmentation was a feature of the cellular response to cerulenin. LoVo cells generated detectable high molecular weight DNA fragments within 12 h after exposure to cerulenin. So, internucleasomal DNA can be detected on agarose gel as a ladder of DNA fragment (Figure 2). To confirm that the cytotoxic effects of cerulenin were mediated via apoptosis, and the treated cells were examined for morphological changes of apoptosis.
Fatty acid synthase played an important role in de novo biosynthesis of fatty acid and consists of two identical subunits each with Mr250000 in cancer cells. Pizer et al[1] reported that OVCAR-3 cells showed a range of elevation fatty acid synthesis in the carcinoma lines, which elevated approximately 10-fold above the level of control skin fibroblasts. The fungal metabolite, cerulenin, (2S-3R)2-3-epoxy-4-oxo-7E. 10Edodecadienamidel, a specific, non-competitive inhibitor of fatty acid synthase, binds covalently to the con densing enzyme site on fatty acid synthase, causing complete inactivation[6,7]. The condensing enzyme site on fatty acid synthase utilizes a biochemically distinctive catalytic mechanism which is not shared by other vital cellular enzymes and is thus well suited to be a therapeutic target. Our data showed that fatty acid synthase inhibitor-cerulenin could suppress the proliferation of LoVo cells in vitro, at the same time, it showed a dose-response relationship. We also found that cerulenin is indeed cytotoxic to cancer cells by clonogenic assay.
It is emphasized that there was a close relationship between apoptosis and proliferation in malignant neoplasm cells[8,9]. In this study, we found that cerulenin could induce LoVo cells to die via apoptosis. Apoptotic cells demonstrated cell shringkage, condensation and fragmentation of chromosomes. Nuclear DNA of apoptotic cells displayed ladder bands characteristic of internucleosomal DNA fragmentation. The data reported here demonstrated that inhibition of fatty acid synthesis inflicted rapid, lethal injury to carcinoma cells via activation of the cell death program. It is similar to that both FAS inhibitors produced rapid, profound inhibition of DNA replication and S phase progression in human cancer cells, meanwhile, apoptosis changes were detected[10].
The strategy of cancer chemotherapy by FAS inhibition was based on the therapeutic index provided by elevated fatty acid synthesis in tumor cells and the apparent tumor preference for the endogenous pathway. Our data proved in principle that the metabolic pathway leading to de novo fatty acid synthesis was selective target for antimetabolic chemotherapy of cancer. Selectivity was conferred by two major factors ① LoVo cells, like some other human cancers, appear to be dependent on de novo fatty acid biosynthesis and therefore sensitive to FAS inhibition; and ② normal tissues (e.g. fibroblasts) seem predominantly toutlize dietary lipid and therefore to be resistant to the cytotoxic effects of FAS inhibition[8]. Possible functional roles for endogenously synthesized fatty acid in cell growth include synthes is of structural lipids for membrane biosynthesis and lipid mediators of intracellular, autocrine or paracrine signals, as well as acylation of other macromolecules. It does appear, however, that for unclear reasons, certain tumors have an apparently obligatory requirement for endogenous fatty acid biosynthesis whereas norm al cells do not, suggesting that inhibition of fatty acid biosynthesis may be a fruitful target for chemotherapy development.
Edited by Ma JY Project supported by the Applied Science Foundation of Jiangsu Provinc e, China, No. BJ97071
| 1. | Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189-1193. [PubMed] |
| 2. | Alo' PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77:474-482. [PubMed] |
| 3. | Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, Kuhajda FP, Hamilton SR. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol. 1997;150:201-208. [PubMed] |
| 4. | Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol. 1996;27:917-921. [PubMed] |
| 5. | Epstein JI, Carmichael M, Partin AW. OA-519 (fatty acid synthase) as an independent predictor of pathologic state in adenocarcinoma of the prostate. Urology. 1995;45:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 159] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976;40:681-697. [PubMed] |
| 7. | Funabashi H, Kawaguchi A, Tomoda H, Omura S, Okuda S, Iwasaki S. Binding site of cerulenin in fatty acid synthetase. J Biochem. 1989;105:751-755. [PubMed] |
| 8. | Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013-2026. [PubMed] |
| 9. | Williams GT. Programmed cell death: apoptosis and oncogenesis. Cell. 1991;65:1097-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 598] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Pizer ES, Chrest FJ, DiGiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58:4611-4615. [PubMed] |