Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.281
Revised: August 26, 1999
Accepted: September 1, 1999
Published online: April 15, 2000
- Citation: Tian XJ, Wu J, Meng L, Dong ZW, Shou CC. Expression of VEGF121 in gastric carcinoma MGC803 cell line. World J Gastroenterol 2000; 6(2): 281-283
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/281.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.281
Vascular endothelial growth factor (VEGF) which is also known as vascular permeability factor (VPF) is a heparin-binding, dimeric polypeptide growth factor and a potent mitogen for endothelial cells. VEGF can stimulate the endothelial cell growth and enhance the motility through its two known receptors flt-1 and KDR[1]. Acting through these receptors, VEGF may stimulate angiogenesis and promote tumor progression. VEGF121, as one of the four VEGF protein isoforms containing the least number of amino acids, has all the biological function of VEGF and is the ideal isoforms for further studying VEGF at molecular levels[2]. In this study, we cloned VEGF121 cDNA from gastric carcinoma cell MGC803 and had it expressed in E. coli bacterial. Further identification of its expression products was carried out with anti-VEGF monoclonal antibodies. This gives new evidence to clarify the source of VEGF in tumor tissues.
Balb/c mice, female, 6-8 wk old, was purchased from the Animal Center of the Chinese Academy of Medical Sciences. HUVEC was obtained from the Academy of Preventive Medical Sciences and cultured in RPMI1640 with 15% fetal cow serum (full growth medium) at 37 °C with 5% CO2. MGC803 cell line was established from a primary poorly differentiated mucoid adenocarcinoma of human stomach. Recombinant human VEGF121 was obtained from the Department of Immunology, Beijing Medical University. 3H-thymidine was purchased from Shanghai Institute of Atomic Energy.
Balb/c mice were immunized subcutaneously with the purified fusion protein GST-VEGF121 and hybridoma cell clones were obtained by traditional hybridoma technology. ELISA was used to screen hybridoma clones with recombinant fusion protein GST-VEGF and GST-P21 as antigen. The clones which reacted with GST-VEGF, but not with GST-P21, were subcloned. After three rounds of subcloning by limited dilution, VEGF121, which had proliferation activity on HUVEC, was used as antigen to select the positive clones. The antibodies were purified through protein A-Sephasrose CL-4B chromatography and their subclasses were measured.
Assay of 3H-thymidine incorporation was used on HUVEC for neutralizing the activity of anti-VEGF121 monoclonal antibody. HUVEC was seeded at a density of 2 × 104 per well of 24 well plates and incubated with full growth medium for 48 h at 37 °C. The cells were then incubated with serum free medium for 24 h, and the testing groups were added with VEGF121 (10 μg/L) and anti-VEGF121 monoclonal antibody at various concentrations. After 30 h culture, 3H-thymidine (37 KBq/mL) was added, and after 6 h, the cells were collected and measured in a liquid scintillation counter.
Total RNA of both cell lines was extracted respectively by TRISOLVTM isolation of RNA kit (GIBCO BRL). First-strand cDNA was synthesized using the SuperscriptTM-II Preamplification System for First Strand cDNA Synthesis Kit (GIBCO BRL) with 5 μg total RNA in a 20 μL reaction volume. Two μL cDNA was used as template in a 100 μL- PCR reaction volume. The primer for VEGF reverse transcription was oligo dT. The cDNA encoding VEGF was amplified using forward primer (5′-GGGGGATCCGCCTCCGAAACCATGAACTT-3′ containing Bam-HI restriction endonuclease site) and reverse primer (5′-CCCGAATTCTCCTGGTGAGAGATCTGGTT-3′, containing Eco-R I restriction endonuclease site), and PCR was carried out in a DNA EngineTM-Peltier Thermal Cycler Model PTC-200 (MT Research Inc, USA) at 95 °C for 5 min first, then at 94 °C for 45 s, 55 °C for 40 s, and 72 °C for 1 min for 36 cycles, followed by 5 min at 72 °C.
The PCR products were purified with DNA purified kit (QIAEGN), digested with Bam-H I/Eco-R I and ligated with fusion protein prokaryotic expression plasmid PGEX-2T. E. coli-XL-1 blue was transformed and the positive clones were selected by restriction endonuclease mapping.
Positive clones were selected and cultured with LB until the OD600 reached to 1.0, then the 5 mmol/L IPTG was added for 4 h culture to induce the protein expression. The expression products were analysed by SDS-PAGE, and Western blot was carried out for further identification of the products.
After three rounds of subcloning and selecting by ELISA with recombinant fusion protein GST-VEGF121 and GST-P21, six clones stably secreting anti-VEGF121 antibodies were obtained. Screened with purified VEGF121, clone 5C5, which showed more specific binding activity to VEGF, was selected for pre paring ascites. The ascites were purified through chromatography with Protein A-Sepharose CL-4B column. Its subclass is IgG2b.
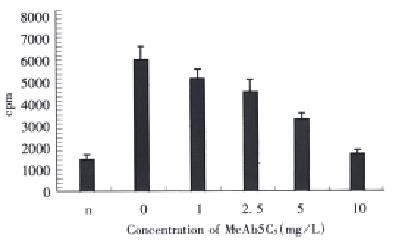
We measured the effects of anti-VEGF antibody 5C5 at various concentrations on HUVEC proliferation induced with VEGF at a concentration of 2 μg/L. The results from analysis of 3H-thymidine incorporation showed that the VEGF antibody 5C5 neutralized the activity of VEGF in a dose-dependent manner and blocked the VEGF-induced cell growth completely at a concentration of 10 mg/L (Figure 1).
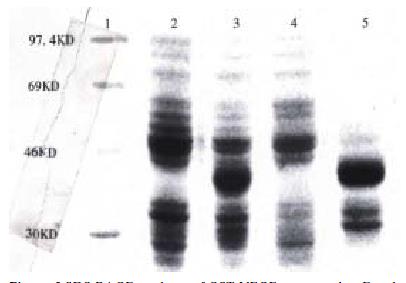
The VEGF121-cDNA was amplified with its specific primers from MGC803 cells. Following 0.8% agarose gel analysis, a clear band about 550 bp, which marched the predicated size, was generated (Figure 2, lane 2). No specific band was obtained from HUVEC (Figure 2, lane 3).
The recombinant PGEX2T-VEGF121 digested with Eco-R I/Bam-H I released a fragment about 550 bp (Figure 2, lane 4). After induced by IPTG, the positive transformed E. coli XL-1 blue can stably express fusion protein GST-VEGF at the molecular weight about 40KD. The proportion of expressed VEGF 121 to total bacterial protein was about 25% and it existed in the inclusion body (Figure 3).
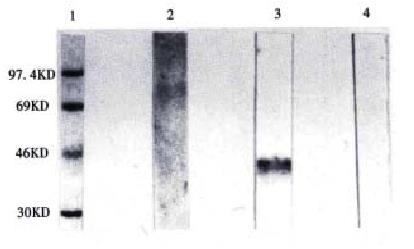
Induced by IPTG, further identification of the expressed product was carried out by Western blot analysis. The results showed that 5C5 can be specifically reacted with denatured GST-VEGF121 (Figure 4).
Inhibition of tumor blood vessel growth is an important research area for tumor biotherapy in recent years. The process of angiogenesis involves stimulation of endothelial cell growth, motility and the release of proteases and the degradation of extracellular matrix. Blocking the overexpression of VEGF in tumor tissues and neutralizing its activities by monoclonal antibodies cast much light on VEGF related tumor therapy[3]. In this study, by using anti-VEGF monoclonal antibodies, we successfully neutralized the VEGF-induced HUVEC growth. These also clearly made the specificity of the prepared monoclonal antibodies.
There are different opinions on which kind of cells in tumor tissues can express VEGF. Wizigmann et al[4] proved that VEGF is mainly expressed by tumor cells. It can bind to its receptors on HUVEC and stimulate cell growth by paracrine ways. However, Plate Hetal[5] discovered that VEGF can be expressed by HUVEC in tumor tissues. Brown[6] said that the HUVECs both in the tumor tissue and the normal tissue can express VEGF. In our study, we demonstrated, by RT-PCR, the expression of VEGF in gastric carcinoma MGC803 cells. We also found, by 3H-thymidine incorporation that the supernate of MGC803 cells can promote the proliferation of HUVEC (data not shown). These suggested that MGC803 can express VEGF, but we failed to amplify VEGF cDNA from HUVEC.
To make clear about which kind of cells in tumor tissue can express VEGF is important for the blocking of its expression at genetic levels. We are preparing human anti-VEGF monoclonal antibodies by the phage display method to obtain the useful anti-VEGF antibodies for further research and its clinical application.
Edited by Ma JY
This work is supported by National Distinguished Young Scientist Fund, 39525021 and State Key Basic Research Program G1998051203
| 1. | Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 916] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 2. | Asano M, Yukita A, Matsumoto T, Matsuo K, Kondo S, Suzuki H. Isolation and characterization of neutralizing monoclonal antibodies to human vascular endothelial growth factor/vascular permeability factor121 (VEGF/VPF121). Hybridoma. 1995;14:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2567] [Cited by in RCA: 2533] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 4. | Wizigmann-Voos S, Breier G, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor and its receptors in von Hippel-Lindau disease-associated and sporadic hemangioblastomas. Cancer Res. 1995;55:1358-1364. [PubMed] |
| 5. | Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 1588] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 6. | Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727-4735. [PubMed] |