Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.263
Revised: August 26, 1999
Accepted: September 1, 1999
Published online: April 15, 2000
- Citation: Sun ZX, Ma QW, Zhao TD, Wei YL, Wang GS, Li JS. Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol 2000; 6(2): 263-265
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.263
The antitumor activity of norcantharidin (NCTD), the demethylated analogue of cantharidin, was studied in the early 1980s in China. NCTD has no side effects on urinary organs which cantharidin has shown and is easier to synthesize, and it can inhibit the proliferation of several tumor cell lines as well as transplanted tumors. Clinical trials with NCTD as a monotherapeutic agent indicated that NCTD had beneficial effects in patients with different kinds of digestive tract cancers, such as primary hepatoma, carcinomas of esophagus and gastric cancer, but no depressive effect on bone marrow cells. NCTD can increase the white blood cell count by stimulating the bone marrow and has some antagonistic effect against leukopenia caused by other agents. The exact cellular and molecular mechanisms of NCTD on tumor cells have not yet been elucidated to date[1-3].
Human hepatoma cell line BEL-7402, as monolayer cultures in RPMI-1640 medium supplemented with 20% FCS, was routinely grown at 37 °C and 5% (V/V) CO2. The response of tumor cells to NCTD was studied during the logarithmic growth phase.
NCTD was synthesized from furan and maleic anhydride via the Diels-Alder reaction. RNaseA and proteinase K were purchased from E. Merck, primary antibody (human Bcl-2 specific, rabbit polyclonal antibody) from Santa Cruz Biotechnology, Inc, secondary antibody (biotinylated anti-rabbit IgG) and horseradish peroxidase streptavidin from Vector Laboratories, Inc, and NP-40 and DAB from Sigma.
Cell suspensions were centrifuged (200 × g, 10 min), and fixed as a pellet in 2.5% glutaraldehyde-1% osmium tetroxide buffered with PBS (pH7.2). The cell samples were dehydrated in a graded ethanol series, embedded in Spury Resin and analyzed with standard procedures.
Apoptotic fragments were isolated as described[4]. After harvesting, the cell samples were washed with PBS and pelleted by centrifugation. The cell pellets were then treated for 10 s with lysis buffer (1% NP-40 in 20 mmol/L EDTA, 50 mmol/L Tris-HCl, pH7.5; 10 μL-/106 cells). After centrifugation for 5 min at 1600 × g, the supernatant was collected and the extraction was repeated with the same amount of lysis buffer. The supernatants were brought to 1% SDS and treated for 2 h with RNaseA (a final concentration of 5 g/L) at 56 °C followed by digestion with Proteinase K (a final concentration of 2.5 g/L) for at least 2 h at 37 °C. After addition of 1/2 volume 10 mol/L ammonium acetate, the DNA was precipitated with 2.5 volume ethanol, dissolved in gel loading buffer, and separated by electrophoresis in 1% agarose gels.
Apoptotic gene bcl-2 product was analyzed by standard SABC procedures. After washed with 0.01 mol/L PBS (pH7.4) three times for 5 min, cells were fixed with PLP (periodate-lysine-paraformal dehyde fixative) for 20 min at room tem perature. Washed with PBS again, and cells covered with 3% H2O2 for 10 min, 0.3% Triton X-100 for 30 min and 3% sheep serum for 40 min before primary antibody was added. Cells were covered with primary antibody (1∶50) overnight at 4 °C. Biotinylated secondary antibodies (1∶200) were reacted for 30 min at room temperature, then incubated cells with horseradish peroxidase streptavidin (1∶400) for 30 min at room temperature. Cells were washed in PBS three times for 5 min between each step. Cells were incubated in DAB solution for 5 min and examined under microscope. Cells were rinsed in distilled water and dehydrated through alcohol and xylene and mounted coverslip using a permanent mount medium for analysis by microspectrophotometer (LEITZ DMRBE, Leica). Three to five sample slides in each group were chosen for analysis and 90 cells in each group were determined for mean light absorbance. The significance of difference between experimental data was validated using the t test.
Approximately 5 × 106 cells were collected, and lysed in 100 μL 2 × electrophoresis loading buffer, boiled for 10 min and electrophoresed through 12.5% SDS-polyacrylamide gels. Proteins were electrotransferred onto the nitrocellulose membrane. Filters were blocked overnight at 4 °C with TBST [10 mmol/L Tris-HCl (pH8.0), 0.15 mol/L NaCl, 0.05% Tween 20] containing 3% nonfat milk. All additional immunostaining steps were performed in TBST at room temperature. Filters were incubated with primary antibody (1∶1000) for 2 h. Biotinylated secondary antibodies (1∶2000) were reacted for 30 min. Immunoblots were reacted with horseradish peroxidase streptavidin (1∶2000) for 30 min. Filters were washed in TBST for 3 times for 20 min between each step and were developed with DAB, and enhanced with H2O2 (0.03%).
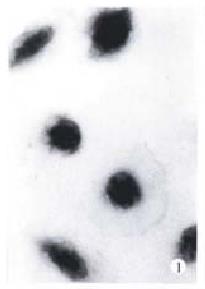
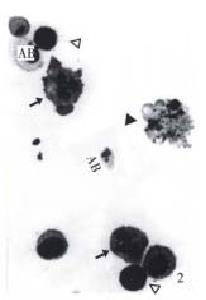
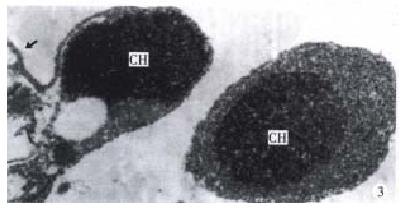
The morphology of human hepatoma BEL-7402 cells changed significantly (Figure 1, Figure 2, Figure 3) after 24 h-10 mg/L NCTD treatment. Some cells condensed and some showed membrane bubbling aspect. Stained with Wright Giemsa, the cells showed M phase arrest and chromosome multipolar distribution. Nuclear chromatin compactions were observed under electron microscopy. At the same time, many membrane-enclosed bodies with cytoplasm and chromatin fragments were formed. These morphological changes are similar to the description of apoptosis[5].
A characteristic DNA “ladder” of apoptosis occurred in NCTD treated groups but did not appear in control groups (Figure 4).
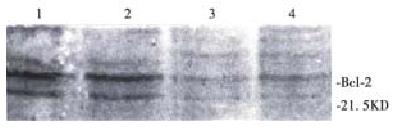
Exist site of protein Bcl-2 presented yellow-brown reactant by immunocytochemical stain. Protein Bcl-2 was not expressed in the negative control group, and cells in control group were stained brown, showing that protein Bcl-2 expressed strongly in tumor cells. Treated with 10 mg/L NCTD for 24 h, the staining was weakened to light yellow-brown between negative control group and control group. The mean light absorbance of cells in the control group was 0.12 ± 0.01 (n = 90), while 0.08 ± 0.01 (n = 90) in treatment group. Compared with control cells, the expressions of Bcl-2 in treatment group were significantly decreased (P < 0.001). The result of Western blot analysis (Figure 5) showed that though protein Bcl-2 had a strong expression in BEL-7402 cells when treated with 10 mg/L NCTD for 24 h, 48 h and 72 h, the expression was weakened sharply.
Apoptosis is an active, inherently programmed phenomenon. Morphologically, it involves rapid condensation and budding of the cell, with the formation of membrane-enclosed apoptotic bodies. A characteristic biochemical feature of the process is double-strand cleavage of nuclear DNA at the linker regions between nucleosomes leading to the production of oligonucleosomal fragments[6]. Obviously, NCTD can inhibit the growth of tumor cells mainly by inducing apoptosis.
Great progress has been made in researches on genetic regulation of apoptosis in recent years[7]. Proto-oncogene bcl-2, might participate in the apoptotic process under some certain circumstances. The expression of Bcl-2 protein in BEL-7402 cells significantly changed in apoptotic process induced by NCTD. With the treatment time of NCTD prolonged, the expression of protein Bcl-2 decreased and paralleled with the apoptotic process. We believe that the decrease of protein Bcl-2 may play an important part in apoptosis of tumor cells induced by NC TD.
In a continuous observation, we found the treated BEL-7402 cells became round and separated from adjoining cells in the early stage, then bubbled, and membrane-enclosed bodies were formed. Stained with Wright-Giemsa, the round and bubbled cells were all presented with M phase arrest, and chromosome breaking, multipolar distribution, chromatin clumping and membrane-enclosed bodies with chromatin fragments forming were observed. All these indicated that the apoptosis induced by NCTD in BEL-7402 cells had a close relationship with tumor cell M phase arrest. More studies on the relationship between apoptosis and tumor cell M phase arrest are in progress.
Edited by Ma JY
| 1. | Wang GS. Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol. 1989;26:147-162. [PubMed] [DOI] [Full Text] |
| 2. | Liu XH, Blazsek I, Comisso M, Legras S, Marion S, Quittet P, Anjo A, Wang GS, Misset JL. Effects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cells. Eur J Cancer. 1995;31A:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Sun ZX, Li JS. Review on proceedings of norcantharidin. Xibei Yaoxue Zazhi. 1998;13:227-229. |
| 4. | Herrmann M, Lorenz HM, Voll R, Grünke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506-5507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 518] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 5. | Bergamaschi G, Rosti V, Danova M, Lucotti C, Cazzola M. Apoptosis: biological and clinical aspects. Haematologica. 1994;79:86-93. [PubMed] |
| 6. | Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 7. | Zeng YY. Advances on research of molecular mechanisms in apoptosis. Zhongguo Kexue Jijin. 1999;13:137-144. |