Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.223
Revised: July 6, 1999
Accepted: July 19, 1999
Published online: April 15, 2000
AIM: To investigate the clinical features of FADD and TRADD expressions in primary hepatocellular carcinoma (HCC) and to determine their relationship with hepatic apoptosis.
METHODS: FADD and TRADD expressions were detected by immunohisto chemistry and hepatic apoptosis were determined by in situ end-labeling (ISEL).
RESULTS: Ten (25.6%) cases of HCC were detected to express FADD protein. The positive rate in HCC is lower than that in non-cancerous adjacent liver tissues (62.5%) (P < 0.05). In those of grade I-II, 8 (38.1%) cases were FADD positive, while only 2/18 (11.1%) cases of grade III-IV had detectable FADD protein (P < 0.05). No relationship was found between FADD expression and other clinical features, such as gender, age, tumor size, differentiation or metastasis. ISEL positive cells can be seen in all cases of HCC. The hepatic apoptosis was associated with FADD expression as more apoptotic cells were detected in those cases which had moderately to strongly positive FADD, as compared with negative or weak positive FADD cases (P < 0.05). No relationship was found between FADD expression and hepatic apoptosis in non-cancerous adjacent liver tissues. Fifteen of 39 (38.5%) cases of HCC were found positive for TRADD protein, and similar positive rate (37.5%) in non-cancerous adjacent liver tissues (P > 0.05). The expression of TRADD is correlated with HCC differentiation, as only 22.2% of moderately to highly differentiated HCC showed positive TRADD protein, while as high as 52.4% of poorly differentiated HCC had TRADD (P < 0.05). No relationship was found between TRADD expression and gender, age, tumor size or grade or metastasis, although 42.9% of HCC of grade I/II showed positive TRADD which was slightly higher than that of grade III/IV (33.3%, P > 0.05). Hepatic apoptosis was not related to TRADD expression in HCC or non-cancerous adjacent liver tissues.
CONCLUSION: Loss of FADD expression plays an important role in HCC carcinogenesis, and expression of TRADD also contributes to HCC development. The cell apoptosis in HCC is associated with FADD expression. However, the expression of TRADD does not correlate well with hepatic apoptosis in HCC.
- Citation: Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol 2000; 6(2): 223-227
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/223.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.223
Apoptosis is an area of intense scientific interest, which encompasses the study of and triggers mechanisms involved in mediating the cell biology of programmed cell death. Deregulation of apoptosis is generally considered as a critical reason for tumorgenesis. In addition to uncontrolled cell proliferation, decreased cell death is also involved in increase of tumor cell population[1]. Fas, TNFα and TGFβ play prominent roles in regulating liver cell apoptosis[2]. Trimerization of the Fas receptor (CD95, APO-1), a membrane bound protein, after binding to Fas ligand, triggers cell death by apoptosis. The main death pathway activated by Fas receptor involves the Fas-associated death domain (FADD) adapter protein that connects Fas receptor to the caspase cascade. Transient expression of a dominant-negative mutant of FADD impairs Fas/Apo1-mediated apoptosis. TNFR-1 recruits and assembles, as a consequence of TNFα binding to TNFR-1, a signaling complex containing a number of death domain (DD)-containing proteins, including the TNFR-associated death domain (TRADD) adapter protein. The subcellular interactions of TNF-R1 and the TRADD adapter protein serving as anchor for the subsequent recruitment of other proteins into the signaling complex that directly lead to cell death or nuclear factor-kappaB (NF- κB) induction[3,4]. Silencer of death domains (SODD), a widely expressed approximately 60-kilodalton protein, was found to be associated with the death domain of TNF-R1 preventing constitutive TNF receptor 1 signaling[5]. Hepatocellular carcinoma (HCC) is one of the tumors known to be resistant to Fas-mediated apoptosis. To elucidate the possible mechanisms of this resistance, we examined the FADD and TRADD protein expression in a series of primary HCC and observed the relationship between FADD and TRADD protein expression and hepatic apoptosis.
The surgically resected specimens employed in this study were obtained from consecutive patients with primary HCC who had undergone potentially curative tumor resection in the Department of General and Hepato-Biliary Surgery, Tongji Hospital during 1996-1997. A cohort of 39 cases was involved in this study. All cases were selected on the basis of availability of frozen material for study and on the absence of extensive chemotherapy-induced tumor necrosis. Materials were com posed of 3 cases of grade I, 18 cases of grade II, 11 cases of grade III, the remaining 7 cases were of grade IV according to TNM system (1987). Twenty-one were poorly differentiated, 9 moderately and 9 well differentiated HCC. There were 34 males and 5 females aged from 24 to 71 years with an average of 46.1 ± 12.5 years. Eight cases of non-cancerous adjacent liver tissues were also included in the study. Routinely processed 4% paraformaldehyde-fixed, paraffin-embedded blocks of containing principal tumor were selected. Serial sections of 5 μm were prepared from the cut surface of blocks at the maximum cross-section of the tumor.
Goat monoclonal antibody that recognizes the human FADD and TRADD protein was Santa Cruz product. StreptAviain- Biotin- enzyme Complex (SABC) kit was purchased from Boster Biotechnology Inc. (Wuhan, China). Briefly, 5 μm tissue sections were deparaffined, rehydrated through a graded series of ethanol, and heated in 0.01 mol/L sodium citrate solution in microwave oven for 15 min. The primary antibody was used at a dilution of 1∶30 (for FADD) and 1∶50 (for TRADD). After incubated overnight at 4 °C, biotinylated antigoat immunolobulin and streptavidin conjugated to horseradish peroxidase were subsequently applied. 3,3’-diaminobenzidine was used for color development, and hematoxylin was employed for counterstaining. Representative tissue sections were immunolabeled with normal goat serum as a negative control for the immunohistochemistry. The intensity of FADD and TRADD immunostaining was scored according to the percentage of positive cells: (-) no positive signal was found; (+) positive cell < 25%; (++) 25%-50%; (+++) > 50%.
Tumor cell apoptosis was identified by DNA fragmentation detection kit (QIA33-Kit, Calbiochem). Briefly, deparaffinized and rehydrated sections were permeabilizated with proteinase K (20 mg/L in 10 mmol/L Tris, pH8.0) for 20 min at room temperature and washed with 1 × TBS (20 mmol/L Tris pH7.6, 140 mmol/L NaCl). After endogenous peroxidases were inactivated by using 30 mL/L hydrogen peroxide for 5 min and washed with 1 × TBS, equilibration buffer was added to each section and incubated at room temperature for 20 min. Terminal deoxynucleotidyl transferase (TDT) enzyme in TDT labeling reaction mixture at a 1∶20 dilution was pipetted onto the sections, followed by 1.5 h incubation at 37 °C. After terminating the reaction by immersing sections into stop solution and washing with blocking buffer for 10 min at room temperature, the anti-digoxingenin-peroxidase was added to the sections. DAB solution was used for color development. Sections were counterstained by methyl green. A positive control was generated covering specimen with DNase I (1 mg/L) for the first procedure. Specific positivet issue sections were used for negative control by substituting distilled water for the TdT in the reaction mixture. Positively stained tumor cells with morphological characteristics of apoptosis were identified using standard criteria, including chromatin condensation, nuclear disintegration and formation of crescentic caps of condensed chromatin at the nuclear periphery. According to, with small modification, Liang’s report[6], the positive ISEL was determined in least five areas at × 400 magnification and as signed to one of the three following categories: (+) only sporadic positive cells were detected; (++) a cluster of apoptotic cells were observed; (+++) positive cells in a large scale or multi-cluster apoptotic cells were seen in representative tissue sections of each individual case.
The association between the variables was assessed using the Chi-square and Fisher exact tests. Differences in frequencies were considered statistically significant if P values less than 0.05.
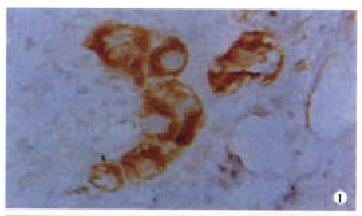
Ten (25.6%) out of 39 cases had detectable FADD protein expression, the positive rate being lower than that of non-cancerous adjacent live tissues (62.5%). The positive signal was predominantly located at cytoplasm (Figure 1). In 18 cases of moderately-well differentiated HCC, the positive rate was slightly higher, however with no statistical significance, than that of poorly differentiated HCC (33.3% vs 19%, P > 0.05). No relationship was found between FADD expression and other HCC clinical features including gender, age, tumor size and metastasis with exception for tumor grade, which showed that 38.1% were positive for FADD in cases of grade I/II and 11.1% in cases of grade III/IV (P < 0.05, Table 1).
| Variables | n | FADD | TRADD | ||
| Positive | % | Positive | % | ||
| Adjacent tissue | 8 | 5 | 62.5a | 3 | 37.5 |
| HCC tissue | 39 | 10 | 25.6 | 15 | 38.5 |
| Age (yr) | |||||
| ≤ 60 | 28 | 6 | 21.4 | 9 | 32.6 |
| > 60 | 11 | 4 | 36.4 | 6 | 54.5 |
| Sex | |||||
| Male | 34 | 9 | 26.5 | 13 | 38.2 |
| Female | 5 | 1 | 20 | 2 | 40 |
| Tumor size (cm) | |||||
| ≥ 5 | 27 | 8 | 29.6 | 10 | 37 |
| < 5 | 12 | 2 | 16.7 | 5 | 41.7 |
| Differentiation | |||||
| Moderately well | 18 | 5 | 27.8 | 4 | 22.2a |
| Poorly | 21 | 5 | 23.8 | 11 | 52.4 |
| TNM grade | |||||
| I/II | 21 | 8 | 38.1a | 9 | 42.9 |
| IV/IV | 18 | 2 | 11.1 | 6 | 33.3 |
| Metastasis | |||||
| yes | 8 | 3 | 37.5 | 4 | 50 |
| no | 31 | 7 | 22.6 | 11 | 35.5 |
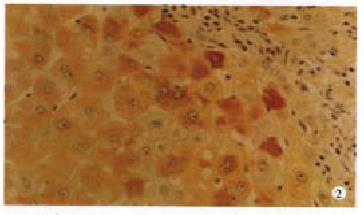
Fifteen cases of HCC were immunolabeled by anti-TRADD antibody. The positive ra te (38.5%) was similar to that of non-cancerous adjacent liver tissues (37.5%, P > 0.05). The TRADD expression selectively restricted to cytoplasm (Figure 2). The intensity of TRADD staining was usually homogeneous within a case tested. Although TRADD was detected in 9 (42.9%) cases of grade I/II, the difference was not statistically significant as compared with that of grade III/IV which showed 33.3% of positive TRADD (P > 0.05). Negative relationship with statistical significance was found between TRADD staining and HCC differentiation, because 52.4% of moderately/highly differentiated HCC were TRADD detected by IHC while only 22.2% of poorly differentiated HCC harbored TRADD protein (P < 0.05). None of the other clinical parameters analyzed in TRADD-positive cases, including gender, age, metastasis and tumor size reached statistical significance (P > 0.05, Table 1). TRADD and FADD coexpression was found in six cases of HCC, but without relationship between TRADD and FADD expression (P = 0.08).
Apoptotic cells and bodies were found in all cases of HCCs examined by in situ end-labeling-(Figure 3) according to the criteria described in Material and Methods. In the cases with moderately-strong positive FADD immunoreaction, more cells underwent apoptosis than those with weak or negative FADD expression (P < 0.05). FADD protein expression was not related to hepatic apoptosis of non-cancerous adjacent liver tissues (P > 0.05), and no significant differences were observed between TRADD expression and apoptosis in either HCC or adjacent liver sample (P > 0.05, Table 2).
| Apoptosis | n | FADD expression | TRADD expression | ||||||
| - | + | ++ | +++ | - | + | ++ | +++ | ||
| HCC | |||||||||
| + | 19 | 17 | 1 | 0 | 1a | 13 | 3 | 1 | 2 |
| ++ | 14 | 10 | 0 | 2 | 2 | 9 | 2 | 2 | 1 |
| +++ | 6 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 |
| Non-cancerous adjacent liver tissues | |||||||||
| + | 6 | 3 | 2 | 1 | 0 | 4 | 2 | 0 | 0 |
| ++ | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| +++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Cancer results from excessive uncontrolled accumulation of cells. This may be the consequence of enhanced cell proliferation or of reduced cell death, or both. Inappropriate hepatic apoptosis is the critical link in liver injury and diseases. In hepatocarcinogenesis, the balance is disturbed between cell proliferation and death, which is precisely controlled in normal liver. Fas ligand and Fas receptor, tumor necrosis factor (TNF) and its receptor, and transforming growth factor β1 (TGF-β1) and its receptor are currently known as well established ligand/receptor interactions causing hepatocyte apoptosis[2]. After binding to Fas ligand, Fas can interact with FADD protein via its death domain, leading to the activation of initiating cysteine proteases of the caspase family, e.g., caspase 8, and trigger apoptosis. TNFα-induced apoptosis need the presence of TRADD protein, which will transduct the signal from the binding of TNFα-with TNF-R1[7]. Thus, loss of FADD and TRADD function will prevent apoptosis by failing to actively initiate caspase.
In our previous study, we found that the expression of Fas/FasL was not correlated with hepatic apoptosis in certain cases of HCC. So we detected the FADD protein expression in HCC. In this study, the FADD protein was expressed in HCC with a lower incidence than in positive cases in noncancerous adjacent liver tissues (25.6% vs 62.5%, P < 0.05). A similar expression pattern of FAD D was observed in a research consisting of 6 HBV-related HCC and 10 HCC cell lines by Shin et al[8], FADD expression was dramatically reduced in both HCC specimens and cell lines, while FAP (for FAS associated protein) which pronounced antiapoptotic effect when overexpressed, increased in HCC. The result suggested that loss of FADD expression plays a critical role in HCC carcinogenesis and development. In addition, we found no significant correlation between FADD expression and HCC clinical parameters including age, gender, tumor size or metastasis, except for tumor grade. The positive rate for FADD was 38.1% of grade I/II, a little higher than that of grade III/IV (11.1%), with statistical significance (P < 0.05).
FADD alone can trigger apoptosis in a FAS-independent way. Kondo et al[9] had used a selected group of malignant glioma cell lines containing negative or low-level Fas protein for study. The results indicated that about 85% of malignant glioma cells, regardless of Fas/APO-1 expression levels, underwent apoptosis after transient transfection with FADD expression vector. The retroviral transfer of FADD gene significantly enhanced the transduction efficiency and effectively inhibited both in vitro and in vivo survival of malignant glioma cells through induction of apoptosis. Chemotherapeutic drugs were reported to induce the accumulation of the FADD adapter molecule in several human cancer cells[10]. It is proposed that FADD expression will contribute to HCC apoptosis. In this study, the apoptotic cells can be seen in all HCC samples and the intense is correlated to the FADD protein level. We found that more apoptotic cells were detected in HCC with moderate to strong FADD expression than those with weak positive or negative FADD. This relationship was not observed in non-cancerous adjacent liver tissues. The results indicate that FADD expression is associated with HCC hepatic apoptosis, but not in the noncancerous liver tissues.
TRADD protein was detected in 15 cases of HCC. The difference of TRADD expression in HCC and noncancerous adjacent liver tissues was not statistically significant. We proposed that the TRADD expression may be related to tumor differentiation of HCC because positive rate in moderately to well differentiated cases (52.4%) was higher than that in the poorly differentiated ones (22.2%). No relationship was observed between TRADD expression and tumor size, metastasis, age or gender of patients. Unlike the expression of FADD, TRADD expression was not significantly related to hepatic apoptosis. In fact, it is believed that in addition to FADD, TRADD can bind to RIP, preventing cell apoptosis via NF-κB dependent and independent pathway[2]. TRADD was also demonstrated to interact with death receptor 6 causing cell apoptosis[11]. No relationship was found between FADD and TRAD D expression, indicating that different mechanisms are involved in the regulation of FADD and TRADD expression.
There have been many studies on the mechanisms by which death receptor triggers apoptosis. Our knowledge of the intracellular signaling mechanism will enable us to improve the therapeutic strategies for the treatment of liver cancer[1].
Edited by Ma JY
| 1. | Wyllie AH. Apoptosis and carcinogenesis. Eur J Cell Biol. 1997;73:189-197. [PubMed] |
| 2. | Faubion WA, Gores GJ. Death receptors in liver biology and pathobiology. Hepatology. 1999;29:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Sun BH, Yang DL, Hao LJ. Proteolytic enzyme in apoptosis signal transduction. Guowai Yixue Fenzi Shengwuxue Fence. 1999;21:1-5. |
| 4. | Kidd VJ. Proteolytic activities that mediate apoptosis. Annu Rev Physiol. 1998;60:533-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 293] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Liang YR, Wang CF, Zhou JH, Peng XZ. Apoptosis of hepatocyte and precancerous lesion of hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:160-162. |
| 7. | Galle PR, Krammer PH. CD95-induced apoptosis in human liver disease. Semin Liver Dis. 1998;18:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Shin EC, Shin JS, Park JH, Kim JJ, Kim H, Kim SJ. Expression of Fas-related genes in human hepatocellular carcinomas. Cancer Lett. 1998;134:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Kondo S, Ishizaka Y, Okada T, Kondo Y, Hitomi M, Tanaka Y, Haqqi T, Barnett GH, Barna BP. FADD gene therapy for malignant gliomas in vitro and in vivo. Hum Gene Ther. 1998;9:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT. Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem. 1999;274:7987-7992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 239] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Pan G, Bauer JH, Haridas V, Wang S, Liu D, Yu G, Vincenz C, Aggarwal BB, Ni J, Dixit VM. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett. 1998;431:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |