Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.216
Revised: December 6, 1999
Accepted: December 25, 1999
Published online: April 15, 2000
AIM: To reveal the inhibitory effects of Curcuma aromatica oil (CAO) on cell proliferation of hepatoma in mice.
METHODS: Two tumor inhibitory experiments of CAO on hepatoma in mice were conducted. The inhibitory effects of CAO on proliferation of hepatoma in mice were evaluated by DNA image cytometry and immunohistochemical staining of proliferating cell nuclear antigen (PCNA).
RESULTS: The tumor inhibitory rates of CAO were 52% and 51% in two experiments, respectively. Compared with those of the saline-treated control groups, both differences were statistically significant (P < 0.01). In the group of mice treated with CAO, the cellular nuclear DNA OD value (249 ± 70), are as (623 μm2± 228 μm2) and DNA (2.38 ± 0.67) index of hepatic carcinomas were significantly lower than those of the control group (430 ± 160, 1073 μm2± 101 μm2 and 4.48 ± 0.71). CAO also could increase diploidy cell rates (29.00% ± 9.34% vs 2.97% ± 5.69%, P < 0.01) and decrease pentaploidy cell exceeding rate (30.04% ± 15.10% vs 70.89% ± 14.94%, P < 0.01). In the group of mice treated with CAO, the labeling indexes of proliferating cell nuclear antigen (PCNA-LI) were 30% ± 4%, which were significantly lower than 40% ± 6% of the control group (P < 0.01).
CONCLUSION: The inhibition of CAO on the growth of hepatoma in mice might be associated with its depression on cellular proliferative activity.
- Citation: Wu WY, Xu Q, Shi LC, Zhang WB. Inhibitory effects of Curcuma aromatica oil on proliferation of hepatoma in mice. World J Gastroenterol 2000; 6(2): 216-219
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/216.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.216
Curcuma aromatica oil (CAO) is a volatile oil extracted from a traditional Chinese herb, Curcuma aromatica Salisb, which exerts various medical activities such as promoting blood circulation to remove blood stasis and treating cancers[1]. It contains several major anti-tumor active ingredients: elemicin, curcumol, curdione, etc[2-5]. Our previous clinical and experimental studies revealed that CAO infused via hepatic artery had ideal therapeutic effects on both the patients with primary liver cancer and the rats with transplanted hepatoma[6,7]. However, the anti-hepatoma mechanisms of CAO remain unresolved. The aim of the current study therefore is to determine the inhibitory effects of CAO on proliferation of hepatoma in mice.
Ten g/L of CAO injections were prepared by the department of preparations in our hospital (Lot. 97041601). The 5-fluorouracilum (5-FU) was produced by Hebei Pharmaceutical Factory (Tianjin, Lot 970277). EMAIL-100 type automated Image Cytometry (ICM) was purchased from Yiming Company (Guangzhou). Immunohistoch -emical LSAB kit (K0679) and monoclonal antibody PC10 (M0879) to proliferative cell nuclear antigen (PCNA) were purchased from Dakopatts, Glostrup, Denmark. The statistics software package named Medical Statistics of China Medical Encyclopedia was provided by Department of Health Statistics of West China Medical University.
Kunming mice, male, weighing 18 g-22 g, were provided by the Laboratory Animal Center, the First Military Medical University. The ascites hepatoma carried by mouse was produced by the Cancer Institute of Sun Yat-Sen University of Medical Sciences.
Two anti-tumor experiments were performed in the Grade II animal laboratory of our hospital. The hepatoma ascites carried by mouse was collected and was diluted with normal saline (NS) to 2 × 1010 cancer cells/L. The diluted hepatoma cells were put on ice during the implantation, and 0.2 mL of hepatoma cells were subcutaneously transplanted to the right axilla in mice. All operations were performed under sterile conditions. After transplanted for 24 h, the mice were numbered and randomly allocated into three groups: negative control group, receiving 0.2 mL NS, ip; positive control group, given 20 mg and 40 mg/kg of 5-FU, ip; and treatment groups, given 100 mg/kg of CAO, ip. The mice were housed in plastic cages under a 12-hour light/dark cycle and fed with a standard pellet diet and distilled water ad libitum. Some of the experimental details are shown in Table 1. At the following day of the termination of treatment, the mice were sacrificed with dislocation of neck. All tumors were peeled off, weighed and the tumor inhibitory rates were calculated. After the second experiment, all tumor specimens were fixed with 100 mL/L neutral formalin for less than 48 h, embedded in paraffin, and cut into 4 μm thick series.
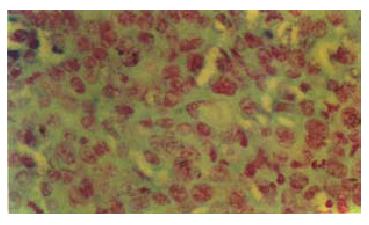
The sections were dewaxed with xylene, rehydrated in decreasing concentrations of ethanol and the DNA staining was ordinarily performed with Foulgen (Figure 1). The cells in the selected visual fields were scanned in super-speed with a television monitor and the DNA histograms were obtained by calculating the integrated optical density with a computer (EMAIL-100). Several visual fields were randomly observed and 50-100 nuclei were detected for each section. The DNA in the normal mouse hepatic cells at G0/G1 phase was employed as the diploid (2C) control. The measurable cellular parameters and paraphrases by ICM were displayed as the follows. DNA optical density (DO) and nuclear area (NA) reflect respectively DNA quantity and nuclei size in hepatoma cells. DNA index (DI) represents the ratio of DNA quantities at G0/G1 phase between hepatoma cells and normal mouse liver cells, 5CER, 2C and 3-5C stand for pentaploidy cell exceeding rate, percentages of diploid cells and of the cells except 5CER and 3C-5C, respectively.
Immunohistochemistry with monoclonal antibody against mouse PCNA was performed by the link streptoavidin-biotin peroxidase method (LSAB kit). Briefly, after sections were deparaffinized with xylene and rehydrated through graded alcohol, they were treated with 30 mL/L H2O2 for 10 min to inactivate end ogenous peroxidase activity. Following a 20-minute blocking step with normal horse serum, the sections were sequentially incubated with PC10 antibody diluted 1∶100 for 30 min, then in anti-rabbit IgG and in avidin-peroxidase complex for 30 min each. The immunoreaction was developed using 3′,3′-diaminobenzidine in the presence of H2O2 to produce a brown precipitate. The sections were counterstained with hematoxylin. Positive and negative controls were included in each experiment. Specifically, for the latter the primary antibody was substituted with nonspecific rabbit IgG.
All of the brown nuclei were regarded as positive for PCNA. The PCNA labeling index (PCNA-LI) was determined by observing more than 2000 nuclei in a few areas of the sections, and the percentage of PCNA-labeled nuclei was used for the analysis. To reduce any interobserver bias, the PCNA-LI of all sections was examined by two experienced pathologists who had no knowledge of the experimental grouping. The mean of the two counts of each observer was considered to be the PCNA-LI.
A statistical evaluation was performed using analysis of variance and t test. All statistical analyses were considered to be significant at a P value of 0.05 or less.
The results from the two experiments showed that the tumor inhibitory rates of CAO on hepatoma were 51.85% and 51.2%, respectively (P < 0.01) (Table 1). Up to the termination of anti-tumor experiment, the body weight in the mice treated with 5-FU tended to decrease, while in the mice treated with CAO, it increased but lighter than that in the negative control group (P < 0.05).
As shown in Table 2, the DO, NA, DI and 5CER in the mice treated with CAO were lower than those in the control mice. CAO could increase the percentage of 2C hepatoma cells and had no significant effect on the percentage of 3.5C cells.
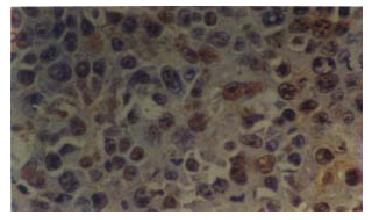
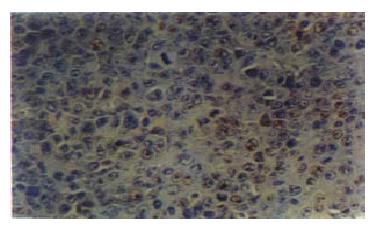
As shown in Table 3 and Figure 2, Figure 3, the percentages of PCNA positive nuclei in the mice treated with either CAO or 5-FU were statistically lower than those in the negative control mice (P < 0.01).
The early literature reported that CAO extracted from Chinese herb, Curcuma aromatica Salisb, could inhibit the growth of various cancer cells in vitro and in vivo[1]. The recent studies showed that CAO was a compound consisting of many kinds of antitumor ingredients such as β-elemene, curcumol, curdione, etc.[2-5]. However, up to date, few researches on anti-hepatoma mechanisms of CAO compound have been conducted. In our previous clinical study, CAO infused via hepatic artery displayed a remarkable therapeutic effect on the patients with primary liver cancer[7]. Our recent animal experimental results revealed that transcatheter infusion of CAO into hepatic artery could significantly inhibit the growth of the implanted hepatoma[6]. The present in vivo study indicated that the tumor inhibitory rates of CAO were around 50%, which coincided with the results reported by the literature. The changes in body weight of the mice treated with CAO might result from their tumors being smaller than those carried by the control group mice and the toxicity of CAO being much less than 5-FU.
Some studies have confirmed that ICM used for the DNA quantitative analysis of cancer cells have advantages of briefness, sensitivity and precision as compared with flow cytometry[8,9]. In the various parameters obtained by ICM, the DO, NA and DI are positively correlated with cellular proliferation. The percentages of aneuploid cells reflect not only cellular malignancy but also the cellular proliferation. ICM has been employed for analyzing various biological features of cancer cell such as proliferation, differentiation, metastasis and prognosis. Zheng et al[10] with ICM revealed that the invasiveness and the metastasis of the hepatic tumors enhanced with the increasing DNA synthesis of hepatocellular carcinoma in the patients with liver cancer. Our present study showed that CAO could significantly decrease the DNA quantity (DO, DI) in the hepatoma cells and shrink the nucleus area. In the untreated mice, the higher 5CER and the lower 2C reflected abnormal increasing proliferation. CAO could decrease the percentage of 5CER cells and increase the percentage of 2C, which showed its inhibition of cellular proliferation.
PCNA functions as a cofactor for DNA polymerase δ are associated with DNA repair in both the S phase and in DNA synthesis[11,12]. Hino et al[13,14] found that the expressions of PCNA were closely related to the malignancies of human hepatocellular carcinoma. With immunohist-ochemical methods, Irene et al[15] and Suehiro et al[16] affirmed that PCNA-LI was correlated with the metastasis and the prognosis of hepatocellular carcinomas. Therefore, PCNA is an important mark for evaluating the proliferation of hepatocellular carcinomas. In the present study, we used LSAB immunohitochemistry to stain the hepatoma nuclei of mice and the positive PCNA cells were at late G1 and early S phases. The results indicated that CAO could remarkably decrease the hepatoma PCNA-LI in mice. Therefore, we assume that the inhibition of CAO on the growth of hepatoma in mice might be associated with its depressing PCNA protein, decreasing DNA-polymerase δ activity and interfering with DNA synthesis. Combining with the ICM results, we have drawn a preliminary conclusion that the depression of CAO on cellular proliferation could contribute to its inhibition on hepatoma growth in mice. The other possible mechanisms of the inhibitory effects of CAO on hepatoma remain further clarification.
Edited by Ma JY
National key project of the 9th 5-year Plan for Medicine and Health, No. 96-906-07-04 and Guangdong provincial natural scientific grants, No.980663.
| 1. | Shi JH, Li CZ, Liu DL. Experimental research on the pharmacology of Curcuma aromatica volatile oil. Zhongyao Tongbao. 1981;6:36-38. |
| 2. | Dong JH, Cheng GB, Hu JH. Isolation and differentiation of g-elemene from volatile oil of curcuma wenyujin and its anti-cancer activity. Zhongcaoyao. 1997;28:13-14. |
| 3. | Yang H, Wang X, Yu L. [The antitumor activity of elemene is associated with apoptosis]. Zhonghua Zhong Liu Za Zhi. 1996;18:169-172. [PubMed] |
| 4. | Chen LB, Zang J, Wang JH, Hu SY, Zhe XY. Synergism in the cytotoxin effects of β-elemene combined with adriamycin or cisplatin on human gastroadenocarcinoma cell line SGC-7901 in vitro. Zhongliu Fangzhi Yanjiu. 1997;24:189-191. |
| 5. | Zhou XJ, Gan XS, Wang LX, Qian JM, Li CS, Meng PL. Inhibition of proliferation and induction of apoptosis of elemene on Himeg cell line. Zhonghua Xueyexue Zazhi. 1997;18:263-264. |
| 6. | Wu WY, Luo YJ, Cheng JH, Chang G, Liu WS, Li RX. Therapeutic effect of Curcuma aramatica oil infused via hepatic artery against transplanted hepatoma in rats. Huaren Xiaohua Zazhi. 1998;6:859-861. |
| 7. | Cheng JH, Wu WY, Liu WS, Chang G, Liu YL, Yang ZG, Li LN, Zhou H. Treatment of 17 cases of patients with primary liver cancer with curcuma aromatica oil infused via hepatic artery. Shijie Huaren Xiaohua Zazhi. 1999;7:92. |
| 8. | Davey DD, Banks ER, Jennings D, Powell DE. Comparison of nuclear grade and DNA cytometry in breast carcinoma aspirates to histologic grade in excised cancers. Am J Clin Pathol. 1993;99:708-713. [PubMed] |
| 9. | Schapers RF, Ploem-Zaaijer JJ, Pauwels RP, Smeets AW, van den Brandt PA, Tanke HJ, Bosman FT. Image cytometric DNA analysis in transitional cell carcinoma of the bladder. Cancer. 1993;72:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Zheng YX, Yu YQ, Xu YD, Lin YQ. Correlation between tumor thormbogenesis in portal vein and DNA content in hepatocellular carcinoma. Zhongliu. 1997;17:145-147. |
| 11. | Lee SH, Hurwitz J. Mechanism of elongation of primed DNA by DNA polymerase delta, proliferating cell nuclear antigen, and activator 1. Proc Natl Acad Sci USA. 1990;87:5672-5676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 163] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Shivji KK, Kenny MK, Wood RD. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992;69:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 583] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 13. | Hino N, Higashi T, Nouso K, Nakatsukasa H, Tsuji T. Apoptosis and proliferation of human hepatocellular carcinoma. Liver. 1996;16:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Hino N, Higashi T, Nouso K, Nakatsukasa H, Urabe Y, Kinugasa N, Yoshida K, Ashida K, Ohguchi S, Tsuji T. Proliferating cell nuclear antigen and grade of malignancy in small hepatocellular carcinoma--evaluation in US-guided specimens. Hepatogastroenterology. 1997;44:245-250. [PubMed] |
| 15. | Ng IO, Lai EC, Fan ST, Ng M, Chan AS, So MK. Prognostic significance of proliferating cell nuclear antigen expression in hepatocellular carcinoma. Cancer. 1994;73:2268-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Suehiro T, Matsumata T, Itasaka H, Yamamoto K, Kawahara N, Sugimachi K. Clinicopathologic features and prognosis of resected hepatocellular carcinomas of varied sizes with special reference to proliferating cell nuclear antigen. Cancer. 1995;76:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |