Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.210
Revised: December 31, 1999
Accepted: January 2, 2000
Published online: April 15, 2000
AIM: To investigate the effects of taxol on SMMC-7721 human hepatoma and its mechanisms.
METHODS: In vitro cell growth was assessed by trypan blue exclusion method. Experimental hepatoma model was established by seeding SMMC-7721 cells subcutaneously into Balb/c (nu/nu) nude mice. In vivo tumor growth was determined by measurement of tumor diameter with Vernier calipers. The syntheses of DNA, RNA and protein were analyzed by incorporation of 3H-thymidine, 3H-uridine and 3H-leucine respectively. Using light and electron microscopes to observe the morphological changes of cells including mitosis and apoptosis.
RESULTS: Taxol was effective against SMMC-7721 human hepatoma cell growth in the ranges of 2.5 nmol/L-10 nmol/L- with mitotic arrest and apoptosis in vitro. DNA, RNA and protein syntheses in cells were also obviously suppressed by in vitro treatment of taxol for 72 h. Taxol at 2.5 nmol/L reduced 3H-thymidine uptake to about 34% of the control value (P < 0.05). Increasing the dose of taxol to 20 nmol/L resulted in a greater decrease in 3H-thymidine incorporation to 60% of the control value (P < 0.01). At a concentration of 20 nmol/L, the 3H-uridine and 3H-leucine uptakes were reduced to 52% (P < 0.05) and 63% (P < 0.01), respectively. In vivo, taxol significantly inhibited SMMC-7721 tumor growth at 10 mg/kg, i.p. once daily for 10 d. A more than 90% decrease in tumor volume was observed by day 11 (P < 0.01) similarly with mitotic arrest and cell apoptosis.
CONCLUSION: Taxol has a marked anticancer activity in SMMC-7721 human hepatoma both in vitro and in nude mice. Its mechanisms might be associated with mitotic arrest, subsequently, apoptosis of the hepatoma cells. No obvious toxicity was observed with in vivo administration of taxol.
- Citation: Yuan JH, Zhang RP, Zhang RG, Guo LX, Wang XW, Luo D, Xie Y, Xie H. Growth-inhibiting effects of taxol on human liver cancer in vitro and in nude mice. World J Gastroenterol 2000; 6(2): 210-215
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/210.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.210
Taxol, a stimulator of tubulin polymerization and microtubule assembly, was initially considered promising because of its cytotoxic activity against several tum or cell lines in vitro[1,2]. Subsequent in vivo studies demonstrated that taxol was effective against a variety of murine tumors and human xenografts[3,4], especially advanced human carcinomas refractory to conventional chemotherapy[5,6]. Now, taxol has emerged as one of the most active anticancer agents in clinic for the therapy of ovarian, breast, and non-small-cell lung cancer[7,8]. Hepatocellular carcinoma (HCC) is among the most common malignancies in the world. The incidence rate is as high as 34 persons per 100000 per year in some high-incidence areas in Asia such as China. However, few studies show that taxol is effective against HCC, especially in vivo[9-13]. Furthermore, HCC is usually insensitive to chemotherapeutic drugs used previously in clinic and there is thus an urgent need for the evaluation of new active drugs against HCC. Therefore, we systematically investigated the activities of taxol against HCC in vitro and in nude mice.
Apoptosis is an active cellular death process induced by physiological or pathological factors[14]. Most anticancer agents cause cell death by apoptosis[15]. Incubation of cells with taxol has been found to result in cell cycle arrest at G2/M phase and cell apoptosis in many tumor cell lines[16,17]. Our previous in vitro studies also show that SMMC-7721 human hepatoma cells treated with taxol exhib it significant G2/M phase arrest and subsequently occurred apoptosis[18]. However, Milross et al[11] reported that taxol caused mitotic arrest but could not induce apoptosis in hepatoma cells. The present studies indicate that taxol can induce mitotic arrest and apoptosis in SMMC-7721 cells not only in vitro but also in vivo.
Taxol was obtained from Shanghai Hualian Pharmaceutical Factory, Shanghai, China. For in vitro experiments, it was dissolved in dimethyl sulfoxide (DMSO) to make stock solution, which was then diluted to desired concentrations with the culture media. The DMSO concentration was kept under 0.001% in all the experiments that did not exert any detectable effect on cell growth or apoptosis. For in vivo experiments, taxol was dissolved in a Cremophor EL vehicle (Cremophor:alcohol = 50:50, v/v) at a concentration of 6 μg/L. This stock solution was further diluted with normal saline (1:5.2, v/v) immediately before injection. 5-Fluorouracil was from Shanghai Puguang Pharmaceutical Factory, Shanghai, China and diluted with normal saline to desired concentration before injection.
SMMC-7721 human liver carcinoma cell line was obtained from Chinese Type Culture Collection (Shanghai Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, China). The cells were grown on monolayer cultures in 37 °C, 5% CO2 incubator in RPMI 1640 media (Gibco, Grand Island, NY) supplemented with 15% heat-inactivated new-born calf serum with or without compounds as described above. At assay time, cells were collected, mixed with an equal volume of phosphate buffer salt solution containing 4 g/L trypan blue dye, and manually counted. Actual cell numbers were calculated by multiplying diluted times compared with initial cell numbers.
Female Balb/c (nu/nu) nude mice (18 g-20 g; 6 wk-8 wk of age) were purchased from Shanghai Institute of Materia Medica, Chinese Academy of Sciences and maintained in our own specific pathogen-free mouse colony. All experiments involving animals were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee.
According to the previous literature[19], the syntheses of DNA, RNA, and protein of cells were measured by quantitating 3H-thymidine, 3H-uricine, and 3H-leucine incorporation, respectively, in the presence and absence of taxol. Briefly, cells were seeded into each well of 96-well Costar flat-bottom culture plates (Costar, Charlotte, NC) at 5 × 103 cells/well in 100 μL of culture medium. After 24 h, taxol was added into each well according to the desired concentrations. Control samples contaned culture medium and DMSO. After 48 h, the cells were incubated with 3H-thymidine, 3H-uridine, or 3H-leucine (1 μCi/well) for 24 h at 37 °C respectively. At the end of the incubation, the plates were immediately placed on ice and frozen at -80 °C. After thawing, 3H-labeled DNA, RNA, or protein were precipitated onto glass fiber discs (Whatman Ltd, Maidstone, UK) using a micro-harvester. For harvesting, the wells were extensively rinsed with distilled water, then, air-dried. The discs were then added to scintillation vials along with 1 mL of scintillant. Radioactivity was measured on a Beckman LS6000 TAβ counter (Beckman Instruments, Irvine, CA).
SMMC-7721 human solitary hepatoma was implanted into the right flank of female nude mice by subcutaneous injection of 2.5 × 106 viable cells. Tumor size (mm3) was evaluated by measurement of two perpendicular diameters with Vernier calipers and using the formula 1/2LW2, where L is the longest diameter and W is the diameter perpendicular to L[20]. Palpable tumors were usually detected after 1 wk and reached 5 mm to 10 mm in diameter by day 10. When tumor grew to 5 mm-10 mm in diameter, the mice were treated with taxol or vehicle by i.p. injection in a volume of 0.2 mL per mouse, once daily for 10 d. Body and internal organ weight of animals were also observed to assess the toxicity of taxol.
In vitro, cells were fixed with 25 g/L glutaraldehyde, and postfixed with 20 g/L osmium tetroxide. After dehydration, the samples were embedded in Epon 812 epoxydic resin, and then sectioned. The sections were routinely stained. In vivo, after the mice were killed by cervical dislocation, tumor tissue was immediately excised and placed in neutral-buffered formalin. The tissue was then imbedded in paraffin blocks from which 4 μm sections were cut and stained with hematoxylin and eosin. The morphologies of cells including mitosis and apoptosis were examined under electron and light microscopes.
The data of in vitro cell growth and the incorporation of 3H-thymidine, 3H-uridine and 3H-leucine into cells were analyzed by Student's t test. Statistical comparisons of tumor volumes were performed by analysis of variance at different time points after initiating treatment.
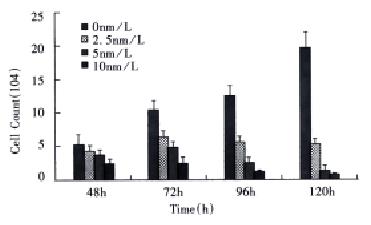
SMMC-7721 cells were treated with different concentrations of taxol for different times and the effect of taxol on the cell growth was examined by trypan blue exclusion method. As shown in Figure 1, the growth of SMMC-7721 cells was markedly inhibited by taxol in the ranges of 2.5 nmol/L-10 nmol/L. Moreover, taxol-induced cytotoxicity was found to be time-dependent when the cells were exposed to the agent for 48 h-120 h.
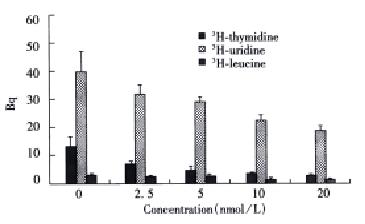
Taxol was significantly effective at inhibiting 3H-thymidine incorporation with dose-dependent relationship when SMMC-7721 cells were continuously exposed to taxol for 72 h. Taxol at 2.5 nmol/L reduced 3H-thymidine uptake to about 34% of the control value (P < 0.05). Increasing the dose of taxol to 20 nmol/L resulted in a greater decrease in 3H-thymidine incorporation to 60% of the control value (P < 0.01) (Figure 2). Taxol also displayed similar dose-dependent efficacy at sup pressing 3H-uridine and 3H-leucine incorporation when the cells were constantly exposed to the drug for 72 h. At a concentration of 20 nmol/L, the 3H-uridine and 3H-leucine uptake was reduced to 52% (P < 0.05) and 63% (P < 0.01), respectively (Figure 2).
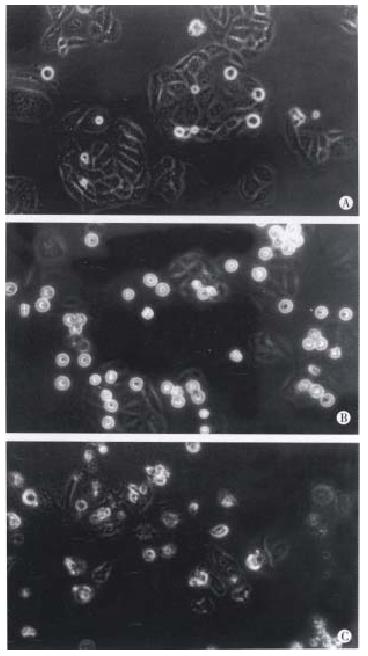
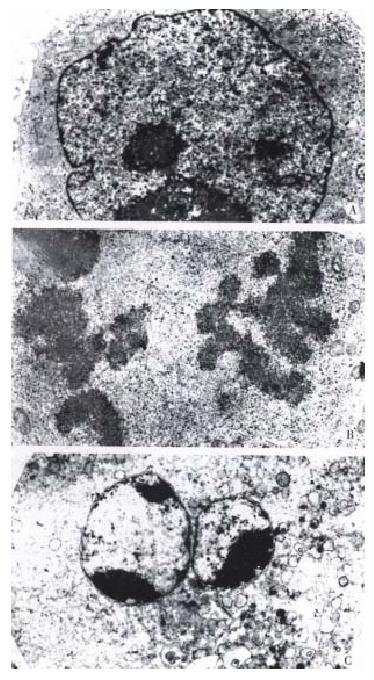
These results show that taxol has significant inhibiting effects on DNA, RNA and protein syntheses of SMMC-7721 cells. The results from light microscope observation indicated that taxol at 10 nmol/L caused obvious morphologic changes of SMMC-7721 cells when compared with untreated cells (Figure 3A). After 24 h treatment (Figure 3B), the cells got round, diopter-enhanced. As time went on for 48 h, cells detached and blebbed (Figure 3C). By analysis with electron microscope, it was found that SMMC-7721 cells exposed to taxol 10 nmol/L for 24 h presented mitotic arrest (Figure 4B), and some cells appeared in pre-apoptotic sign as compared with untreated cells (Figure 4A). After cells were treated by taxol for 48 h, the nuclei were shrank, and the chromatin was condensed to form high density clumps, which were located along the nuclear envelope (Figure 4C), or formed regularly shaped crescents at the nuclear edges. It is suggested that taxol could induce typical characteristic morphological changes of mitotic arrest and apoptosis of cells.
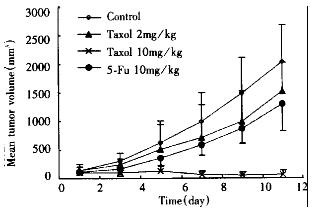
Nude mice bearing SMMC-7721 tumors were treated with different doses of taxol. The taxol response of SMMC-7721 human hepatoma depicted as tumor growth curves in Figure 5 showed that tumors were significantly responsive to taxol when treated at 10 mg/kg, i.p. once daily for 10 d, although treatment with 2 mg/kg proved less effective. Tumor regression was evident by day 3 after taxol treatment (P < 0.05), and a greater than 90% decrease in tumor volume was observed by day 11 (P < 0.01). In contrast, the tumors in animals that only received corresponding solvent continued exponential growth. Mice treated with low or high doses of taxol did not display a body weight loss by day 11 (P > 0.05). No difference of internal organ weight was found between treated and control groups. No obvious other toxicity was observed in mice receiving either 2 mg/kg or 10 mg/kg taxol for 10 d.
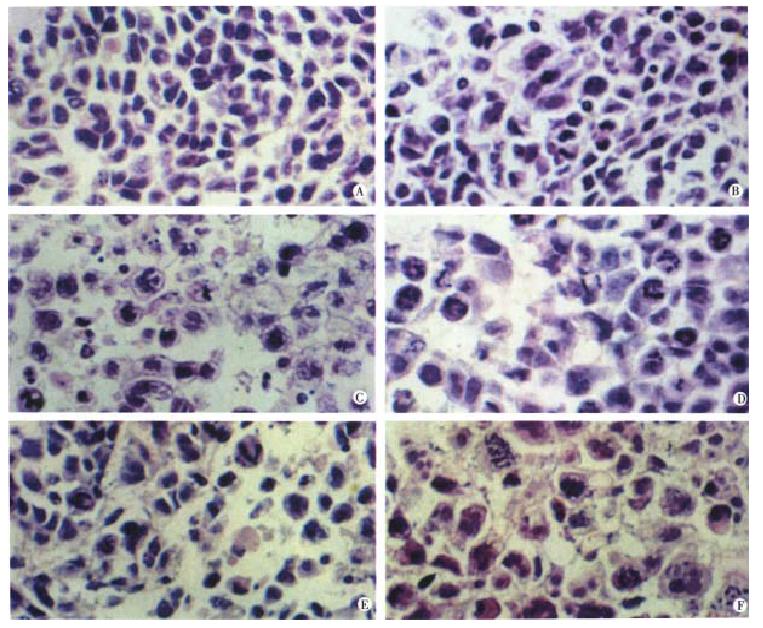
Animals were sacrificed at various times among 24 h and 120 h after administrating taxol. As seen in Figure 6, taxol treatment caused marked morphological changes in SMMC-7721 tumor. Compared with control (Figure 6A), slight mitotic arrest and apoptosis of hepatoma cells were observed in mice with 2 mg/kg taxol treatment for 10 d (Figure 6B). However, most cells exhibited the characteristic appearance of agent-induced apoptosis after treated with 10 mg/kg taxol for 10 d (Figure 6C). These results suggested that taxol-induced mitotic arrest and apoptosis of human hepatoma cells might be dose-dependent. Time-effect analysis indicated that taxol at 10 mg/kg, i.p. for 24 h caused mitotic arrest of most tumor cells with slight apoptosis (Figure 6D). By 48 h, apoptosis became the dominant morphological features (Figure 6E). This phenomenon was more significant after 120 h treatment than ever before (Figure 6F). Apoptotic cells were characterized by overall shrinkage and homogeneous dark basophilia. Frequently, several small apoptotic fragments were encountered in close proximity. On the basis of size and clustering, such fragments were considered to represent the remains of a single cell or apoptotic body.
This study has demonstrated that taxol is significantly effective against SMMC-7721 human hepatoma growth both in vitro and in vivo. In most reports the arrest of cells in G2/M phases and cell apoptosis caused by taxol are responsible for its anticancer activities[21,22]. We have also found that taxol causes SMMC-7721 cell mitotic arrest and apoptosis in vitro and in vivo. It suggests that the anti-hepatoma effects of taxol are related to its mitotic arrest and cell apoptosis. Emphasis has been placed on the anti-hepatoma effects of taxol in vivo. Previous studies showed that taxol had no significant anticancer effect in HCC patients[13]. Others reports also suggested no statistically significant hepatoma growth inhibition in mice treated with taxol at 40 mg/kg, i.v.[11]. The results presented herein indicate that taxol at 10 mg/kg, i.p. once daily for 10 d has significant inhibiting activity on HCC with more than 90% suppressive percentage and without any obvious toxicity. It is inferred that taxol may cause hepatoma growth inhibition at low dose and continuous administration. Our present studies also clearly demonstrate that taxol causes profound mitotic arrest and apoptosis. A time-course microscopic analysis of tumor cell mitosis and apoptosis in nude mice treated with taxol revealed that mitotic arrest occurred previously to apoptosis. Development of apoptosis lagged several hours behind mitotic arrest. Moreover, either the mitotic arrest or apoptosis of hepatoma cells induced by taxol was clearly dose and time- dependent. These data have important therapeutic implications in HCC.
In conclusion, this is the first report of the anti-hepatoma effect of taxol, its mechanisms, and the relationship between the mitotic arrest and apoptosis both in vitro and in vivo.
Edited by You DY
Proofread by Ma JY
Presented at the 4th National Liver Cancer Meeting of the Chinese Cancer Foundation in Chengdu, Sichuan, November 29 - December 1, 1999.
| 1. | Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325-2327. [PubMed] |
| 2. | Douros J, Suffness M. New natural products of interest under development at the National Cancer Institute. Cancer Chemother Pharmacol. 1978;1:91-100. [PubMed] |
| 3. | Riondel J, Jacrot M, Picot F, Beriel H, Mouriquand C, Potier P. Therapeutic response to taxol of six human tumors xenografted into nude mice. Cancer Chemother Pharmacol. 1986;17:137-142. [PubMed] |
| 4. | Riondel J, Jacrot M, Nissou MF, Picot F, Bériel H, Mouriquand C, Potier P. Antineoplastic activity of two taxol derivatives on an ovarian tumor xenografted into nude mice. Anticancer Res. 1988;8:387-390. [PubMed] |
| 5. | Rowinsky EK, Donehower RC. Taxol: twenty years later, the story unfolds. J Natl Cancer Inst. 1991;83:1778-1781. [PubMed] |
| 6. | Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332:1004-1014. [PubMed] |
| 7. | Sandercock J, Parmar MK, Torri V. First-line chemotherapy for advanced ovarian cancer: paclitaxel, cisplatin and the evidence. Br J Cancer. 1998;78:1471-1478. [PubMed] |
| 8. | Ferrante K, Winograd B, Canetta R. Promising new developments in cancer chemotherapy. Cancer Chemother Pharmacol. 1999;43 Suppl:S61-S68. [PubMed] |
| 9. | Lui WY, Chang YF, Li LL, Ho LK, Su TL, Chen JY, Liu TY, P'Eng FK, Chi CW. Differential paclitaxel-induced cytotoxicity in rodent and human hepatoma cell lines. Anticancer Res. 1998;18:3339-3345. [PubMed] |
| 10. | Gagandeep S, Novikoff PM, Ott M, Gupta S. Paclitaxel shows cytotoxic activity in human hepatocellular carcinoma cell lines. Cancer Lett. 1999;136:109-118. [PubMed] |
| 11. | Milross CG, Mason KA, Hunter NR, Chung WK, Peters LJ, Milas L. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J Natl Cancer Inst. 1996;88:1308-1314. [PubMed] |
| 12. | Strumberg D, Erhard J, Harstrick A, Klaassen U, Müller C, Eberhardt W, Wilke H, Seeber S. Phase I study of a weekly 1 h infusion of paclitaxel in patients with unresectable hepatocellular carcinoma. Eur J Cancer. 1998;34:1290-1292. [PubMed] |
| 13. | Chao Y, Chan WK, Birkhofer MJ, Hu OY, Wang SS, Huang YS, Liu M, Whang-Peng J, Chi KH, Lui WY. Phase II and pharmacokinetic study of paclitaxel therapy for unresectable hepatocellular carcinoma patients. Br J Cancer. 1998;78:34-39. [PubMed] |
| 14. | Kroemer G, Petit P, Zamzami N, Vayssière JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277-1287. [PubMed] |
| 15. | Solary E, Bertrand R, Pommier Y. Apoptosis induced by DNA topoisomerase I and II inhibitors in human leukemic HL-60 cells. Leuk Lymphoma. 1994;15:21-32. [PubMed] |
| 16. | Torres K, Horwitz SB. Mechanisms of Taxol-induced cell death are concentration dependent. Cancer Res. 1998;58:3620-3626. [PubMed] |
| 17. | Yu D, Jing T, Liu B, Yao J, Tan M, McDonnell TJ, Hung MC. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell. 1998;2:581-591. [PubMed] |
| 18. | Yuan JH, Wang XW, Luo D, Xie Y, Xie H. Anti-hepatoma activity of taxol in vitro. Acta Pharmacol Sin. 2000;21:450-454. [PubMed] |
| 19. | Richardson DR, Milnes K. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents II: the mechanism of action of ligands derived from salicylaldehyde benzoyl hydrazone and 2-hydroxy-1-naphthylaldehyde benzoyl hydrazone. Blood. 1997;89:3025-3038. [PubMed] |
| 20. | Bullard DE, Schold SC, Bigner SH, Bigner DD. Growth and chemotherapeutic response in athymic mice of tumors arising from human glioma-derived cell lines. J Neuropathol Exp Neurol. 1981;40:410-427. [PubMed] |
| 21. | Roth W, Wagenknecht B, Grimmel C, Dichgans J, Weller M. Taxol-mediated augmentation of CD95 ligand-induced apoptosis of human malignant glioma cells: association with bcl-2 phosphorylation but neither activation of p53 nor G2/M cell cycle arrest. Br J Cancer. 1998;77:404-411. [PubMed] |
| 22. | Ling YH, Consoli U, Tornos C, Andreeff M, Perez-Soler R. Accumulation of cyclin B1, activation of cyclin B1-dependent kinase and induction of programmed cell death in human epidermoid carcinoma KB cells treated with taxol. Int J Cancer. 1998;75:925-932. [PubMed] |