Published online Dec 15, 1999. doi: 10.3748/wjg.v5.i6.541
Revised: August 24, 1999
Accepted: September 22, 1999
Published online: December 15, 1999
- Citation: Lu SJ, Liu YQ, Lin JS, Wu HJ, Sun YH, Tan YB. VIP immunoreactive nerves and somatostatin and serotonin containing cells in Crohn’s disease. World J Gastroenterol 1999; 5(6): 541-543
- URL: https://www.wjgnet.com/1007-9327/full/v5/i6/541.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i6.541
With the progress of the studies on neuroendocrine and immunology in the gastrointestinal tract, it has been recognized that the intestinal neuroendocrine system and the immune system can influence and modulate each other and a neuroendocrine immunomodulation network in the intestine has been established[1]. Neuropeptides, such as vasoactive intestinal peptide (VIP), substance P (SP), somatostatin (SS) etc. are widely distributed in the gastrointestinal tract and they play an important role in the immunomodulation of the intestinal mucosa.
Surgical specimens were obtained from ileum of 25 cases of CD (25 ileum) and 10 normal subjects (from sudden death and who received surgery for intestinal neoplasm). The specimens were fixed in 10% formalin and embedded in paraffin. Sections with thickness of 4ìm were made continuously.
Immunohistochemical staining was carried out by ABC method. Antibodies included rabbit polyclonal antibodies to VIP ( 1:400 ), NSE ( 1:200 ), S-100 protein ( 1:400 ), SS ( 1:200 ), serotonin ( 1:600 ) (Dako Co.) and ABC Kit.
Olympus CH light microscopy with occular linear micrometer and double quardral grid test system C64 (from the Academy of Military Medical Sciences) were used to measure immunoreactive neurons and nerve fibers in 10 × 40 high power field. The point count method was used[2]. Fifteen neuron cells (with nucleus) were examined at random, the maximal diameter of each was measured and the values were averaged. The total length of all immunoreactive nerve fibers was measured separately in the mucosa and the length was estimated per unit area ( μm/μm ) which was expressed as linear density ( Lv% ). In the submucosa and the muscle layers, the total volume of the immunoreactive neurons together with the nerve fibers was measured and the volume was estimated per unit which was expressed as volume density ( Vv% ). The numbers of SS, serotonin immunoreactive cells and a rgyrophil cells in the mucosa were counted at random in 10 high power fields of each case. The results averaged.
Results were analysed by t and t’test.
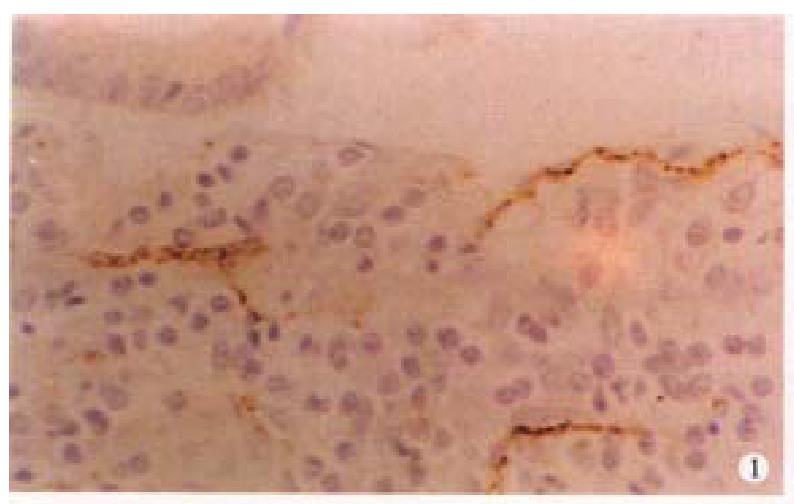
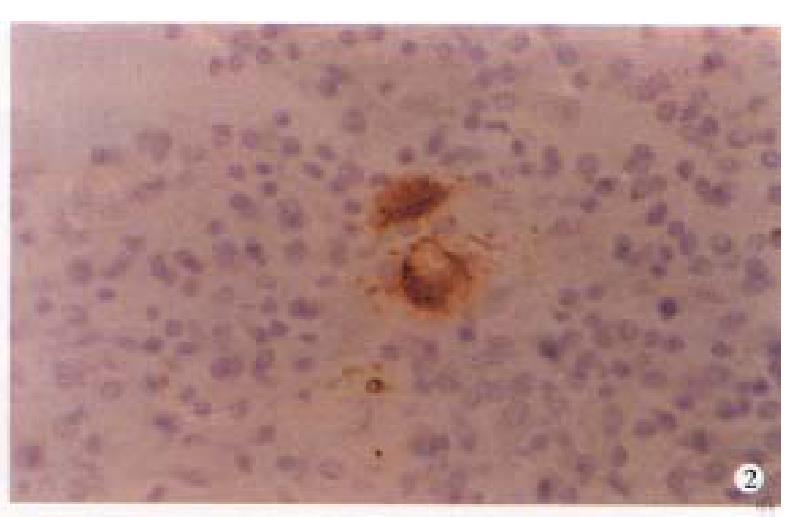
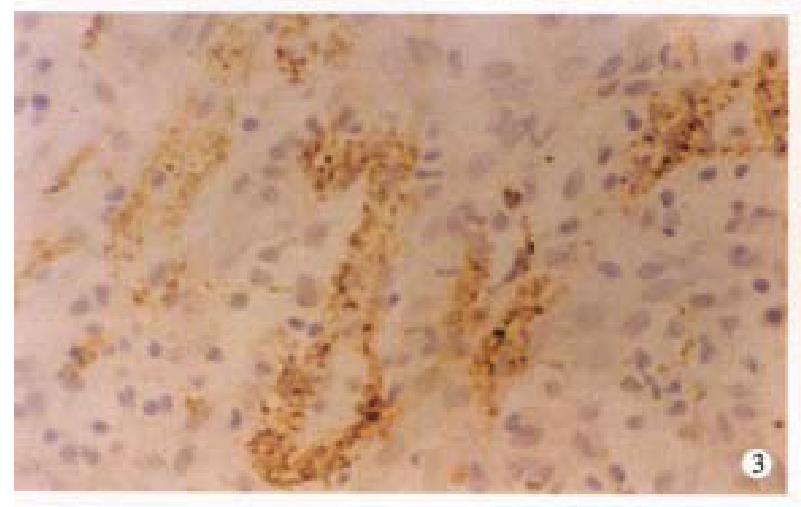
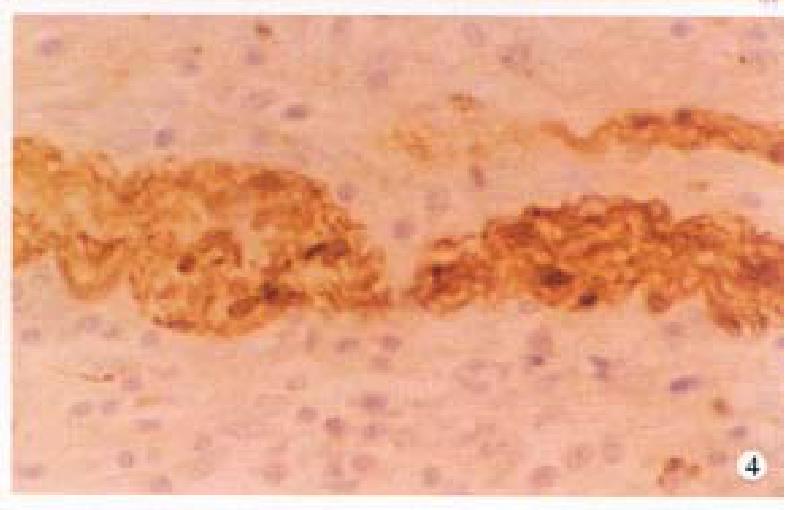
In CD, the VIP-IR and NSE-IR nerve fibers in the mucosa of ileum were markedly increased. They were deeply stained, coarse, irregular ( Figure 1 ) and the linear densities were significantly raised (P < 0.01, P < 0.01, respectively, Table 1). In the submucosa, the VIP-IR neurons were hypertrophial (P < 0.01, Figure 2 ). VIP-IR, S-100 protein IR and NSE-IR nerve fibers were all remarkably increased. They were coarse, thickened and irregular (Figure 3). The neuron and nerve fiber volume density of each immunor eactive type stated above was significantly increased ( P < 0.01, respectively). In the muscle layers the volume density of neurons and nerve fibers containing NSE or S-100 protein was also significantly increased (P < 0.01, respectively, Figure 4), but the changes in VIP-IR neurons and nerve fibers were unremarkable.
| Item | CD | Control | P | ||
| n | -x±s | n | -x±s | ||
| VIP | |||||
| Mucosa Lv | 25 | 3.6 ± 1.2 | 10 | 2.0 ± 0.8 | < 0.01 |
| Submucosa Vv | 25 | 2.0 ± 1.1 | 10 | 0.9 ± 0.8 | < 0.01 |
| Submucosal neuron (diameter, μm) | 25 | 21.1 ± 5.6 | 10 | 13.1 ± 2.0 | < 0.01 |
| Myenteric plexus Vv | 25 | 3.2 ± 1.8 | 10 | 2.6 ± 1.4 | < 0.01 |
| NSE | |||||
| Mucosa Lv | 25 | 3.4 ± 0.7 | 10 | 1.9 ± 1.4 | < 0.01 |
| Submucosa Vv | 25 | 5.1 ± 2.2 | 10 | 1.3 ± 0.5 | < 0.01 |
| Myenteric plexus Vv | 25 | 11.1 ± 2.6 | 10 | 5.1 ± 1.2 | < 0.01 |
| S-100 protein | |||||
| Submucosa Vv | 25 | 3.9 ± 1.0 | 10 | 2.3 ± 0.4 | < 0.01 |
| Myenteric plexus Vv | 25 | 9.4 ± 2.3 | 10 | 5.7 ± 1.0 | < 0.01 |
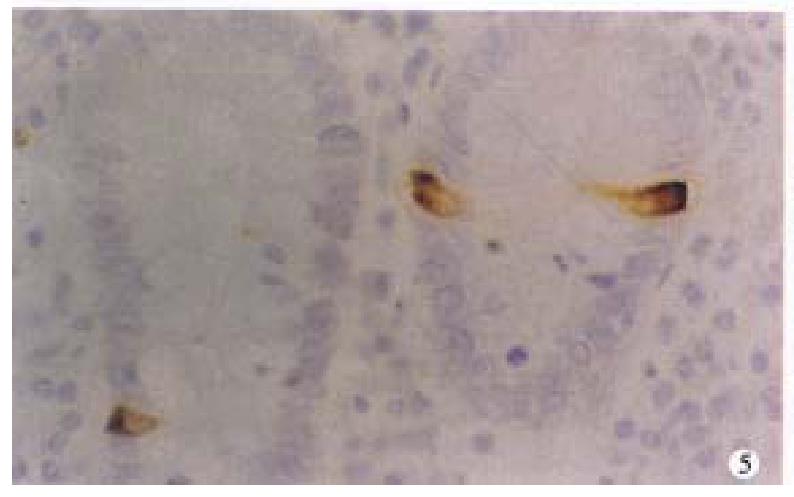
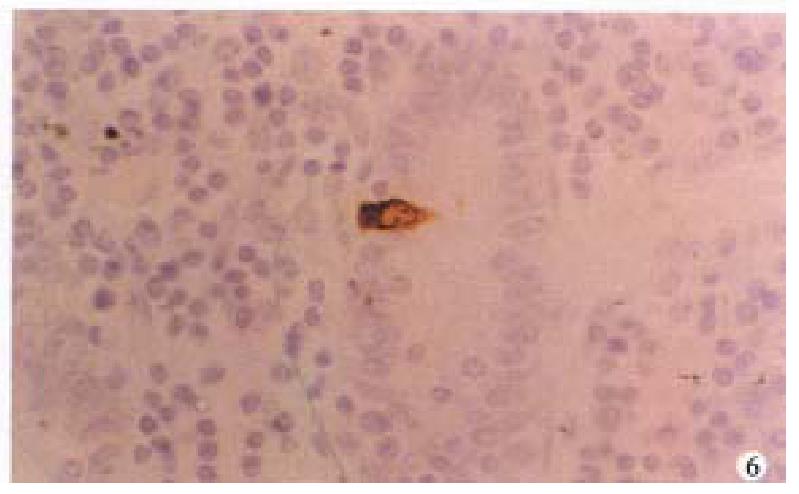
In CD, argyrophil and serotonin-IR cells in the ileal mucosa around the lesions were significantly reduced (P < 0.01, respectively, Figure 5). SS- IR cells were decreased (P < 0.01, Figure 6), Table 2
In CD, there had been several reports about the morphological alterations in the enteric nervous system. Dvorak et al[3] described the changes in enteric nervous system in the surgical specimens from patients with CD including proliferation and focal necrosis of nerve fibers and hypertrophy of neurons in the myenteric plexus. Bishop et al[4] and Sj-lund et al[5] assessed the alterations of VIP-IR enteric nerve fibers in CD observation and counting. In the study, we used the histomorphometric analysis to obtain objective information about the changes in the immunoreactive enteric nervous system in CD. We found that in the submucosal plexus the VIP containing neurons were mar kedly hypertrophied, the linear density of VIP-IR nerve fibers in the mucosa and the VIP-IR neuron nerve fiber volume density in submucosa were significantly increased. The results were consistent with that of Bishop et al[4] and O’Morain et al[6] who measured by immunohistochemistry and radioimmuno assay, but different from that by Sj-lund et al and Koch. By immunohistoche mistry, Sj-lund et al found that in unaffected muscle layer of the ileum and the affected muscle layer of colon, the VIP-IR nerve fibers were reduced. The coarse VIP-IR nerve fibers were more frequently observed in the affected mucosa of ileum and in the affected muscle layers of the colon than those in the control. El-Sathy et al[7] reported that the areas of the argyrophil cells as well as those immunoreactive to chromogranin A and serotonin were significantly increased in both patients with UC and CD, compar ed with those in the controls. In patients with CD, the areas of polypeptide YY (PYY) and pancreatic polypeptide (PP) immunoreactive cells were significantly reduced. In this study, we also found that SS and serotonin containing cells and argyrophil ones were reduced in CD. These diversities among different investig ators may be due to the various locations selected the difference in degree of activity and the methods used.
This paper shows that VIP immunoreactive neurons and nerve fibers are increased whereas the immunoreactive cells containing somatostatin and serotonin are reduced, suggesting that there be abnormalities in neuroendocrine system in CD which may play an important role in the pathogenesis of CD.
Edited by Xie-Ning Wu
Proofread by Qi-Hong Miao
| 1. | Shanahan F, Anton P. Neuroendocrine modulation of the immune system. Possible implications for inflammatory bowel disease. Dig Dis Sci. 1988;33:41S-49S. [PubMed] |
| 2. | Zheng F. Stereocytomorphometry. 1st Ed. Beijing: Beijing Medi-cal University and Union Medical College United Press 1990; 18-20,146-147. |
| 3. | Dvorak AM, Osage JE, Monahan RA, Dickersin GR. Crohn's disease: transmission electron microscopic studies. III. Target tissues. Proliferation of and injury to smooth muscle and the autonomic nervous system. Hum Pathol. 1980;11:620-634. [PubMed] |
| 4. | Bishop AE, Polak JM, Bryant MG, Bloom SR, Hamilton S. Abnormalities of vasoactive intestinal polypeptide-containing nerves in Crohn's disease. Gastroenterology. 1980;79:853-860. [PubMed] |
| 5. | Sjölund K, Schaffalitzky OB, Muckadell DE, Fahrenkrug J, Håkanson R, Peterson BG, Sundler F. Peptide-containing nerve fibres in the gut wall in Crohn's disease. Gut. 1983;24:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | O'Morain C, Bishop AE, McGregor GP, Levi AJ, Bloom SR, Polak JM, Peters TJ. Vasoactive intestinal peptide concentrations and immunocytochemical studies in rectal biopsies from patients with inflammatory bowel disease. Gut. 1984;25:57-61. [PubMed] |
| 7. | El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 5.8] [Reference Citation Analysis (0)] |